Metallic non-magnetic allotrope of iron or solid solution of iron with an alloying element
Not to be confused with Austinite.
| Become |
|
| Microstructures |
|
| Classes |
|
| Other iron-based materials |
|
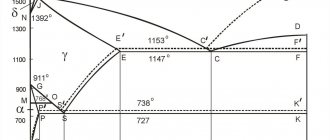
Iron-carbon phase diagram showing the conditions under which austenite
(γ) is stable in carbon steel. Iron allotropes; alpha iron and gamma iron
Austenite
, also known as
gamma-phase iron
(
γ-Fe
), is a metallic non-magnetic allotrope of iron or a solid solution of iron, with an alloying element.[1] In ordinary carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other steel alloys have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902);[2] it exists at room temperature in some stainless steels due to the presence of nickel stabilizing austenite at lower temperatures.
Iron allotrope
From 912 to 1394 °C (1674 to 2541 °F), alpha iron undergoes a phase transition from the body-centered cubic (BCC) to face-centered cubic (FCC) configuration of gamma iron, also called austenite. It is just as soft and ductile, but can dissolve significantly more carbon (up to 2.03% by weight at 1,146 °C (2,095 °F)). This gamma form of iron is present in the most commonly used types of stainless steel[ citation needed
] for the manufacture of equipment for hospitals and public catering.
Material
Austenization
means heating iron, iron-based metal or steel to a temperature at which the crystal structure changes from ferrite to austenite.[3] The more open austenite structure is then able to absorb carbon from the iron carbides in the carbon steel. Incomplete initial austenitization may leave undissolved carbides in the matrix.[4]
For some ferrous metals, iron-based metals and steels, the presence of carbides may occur during the austenitization stage. The term commonly used for this is two-phase austenitization.
.[5]
Outtempering
Main article: Autempering
Outtempering is a hardening process that is used on an iron base. metals to improve mechanical properties. The metal is heated to the austenitic region of the iron-cementite phase diagram and then quenched in a salt bath or other heat-sinking medium at 300–375 °C (572–707 °F). In this temperature range, the metal is annealed until the austenite transforms into bainite or ausferrite (bainitic ferrite + high-carbon austenite).[6]
By varying the austenitizing temperature, different desired microstructures can be achieved during the austenitizing process.[7] A higher austenitization temperature can result in a higher carbon content in the austenite, while a lower temperature results in a more uniform distribution of the hardened structure.[7] The carbon content in austenite has been established depending on the time of austenitic treatment.[8]
Austenite hardness
The hardness of austenite is influenced by various factors, primarily the content of dissolved carbon (and other alloying elements that form substitutional solid solutions), therefore there cannot be a clear and unique value of austenite hardness (only the order of values of austenite hardness is known). Therefore, austenite hardness values are usually indicated within a certain range, and therefore we find slightly different austenite hardness values in different sources. For example, according to [5], the Brinell hardness of austenite is 160-200 HB.
During metallographic analysis in each specific case (alloy, casting), it is desirable to determine the hardness of austenite experimentally, collecting additional statistical information (see Hardness, Microhardness).
©ICM (www.modificator.ru)
Behavior in plain carbon steel
As the austenite cools, carbon diffuses out of the austenite and forms carbon-rich iron carbide (cementite) and leaves behind carbon-poor iron carbide. ferrite. Depending on the composition of the alloy, a layer of ferrite and cementite called pearlite may form. If the cooling rate is very high, the carbon does not have time to diffuse, and the alloy may experience large lattice distortion known as martensitic transformation in which it transforms into martensite, a body-centered tetragonal structure (BCT). The cooling rate determines the relative proportions of martensite, ferrite and cementite and therefore determines the mechanical properties of the resulting steel, such as hardness and tensile strength.
The high cooling rate of thick sections will cause a sharp temperature gradient in the material. The outer layers of a heat-treated part will cool faster and shrink more, causing stretching and heat staining. At high cooling rates, the material will change from austenite to martensite, which is much harder and will crack at much lower strains. Volume changes (martensite is less dense than austenite)[9] can also create stress. The difference in strain rates between the inside and outside of the part can cause cracks to develop in the outside, forcing the use of lower quenching rates to avoid this. By alloying steel with tungsten, the diffusion of carbon is slowed down, and the transformation into the BCT allotrope occurs at lower temperatures, which avoids cracking. It is believed that this material has increased hardenability. Tempering after quenching, part of the brittle martensite is transformed into tempered martensite. If a low hardenability steel is quenched, a significant amount of austenite will remain in the microstructure, causing the steel to experience internal stresses that make the product prone to sudden failure.
Properties of austenitic steels and where they are used
The very state of iron in the Y-phase (austenite) is unique, thanks to which the metal is heat-resistant (+850 ºC), cold-resistant (-100 ºC and below t), capable of providing corrosion and electrochemical resistance and other important properties, without which many would be unthinkable technological processes in:
- oil refining and chemical industries;
- medicine;
- space and aircraft construction;
- electrical engineering.
Heat resistance is the property of steel not to change its technical properties at critical temperatures over time. Fracture occurs when the metal is unable to resist dislocation creep, i.e., the displacement of atoms at the molecular level. Softening gradually occurs, and the aging process of the metal begins to occur faster and faster. This occurs over time at low or high temperatures. So, the extent to which this process extends over time is the metal’s ability to resist heat.
Corrosion resistance is the ability of a metal to resist destruction (dislocation creep) not only over time and at cryogenic and high temperatures, but also in aggressive environments, that is, when interacting with substances that actively react with one or more component elements. There are 2 types of corrosion:
- chemical - oxidation of metal in environments such as gas, water, air;
- electrochemical - dissolution of a metal in acidic environments containing positively or negatively charged ions. When there is a potential difference between the metal and the electrolyte, inevitable polarization occurs, leading to partial interaction of the two substances.
Cold resistance - the ability to maintain structure at cryogenic temperatures over a long period of time. Due to the distortion of the crystal lattice, the structure of cold-resistant steel is capable of taking on the structure inherent in ordinary low-alloy steels, but at very low temperatures. But these steels have one drawback - they can have full properties only at subzero temperatures; t - ≥ 0 is unacceptable for them.
Stabilization
The addition of certain alloying elements, such as manganese and nickel, can stabilize the austenitic structure, making low alloy steels easier to heat treat. In the extreme case of austenitic stainless steel, the much higher alloy content makes this structure stable even at room temperature. On the other hand, elements such as silicon, molybdenum, and chromium tend to destabilize austenite, increasing the temperature of the eutectoid.
Austenite is only stable above 910 °C (1670 °F) in the metal mass. However, fcc transition metals can be grown on face-centered cubic (fcc) or cubic diamond.[12] Epitaxial growth of austenite on the (100) diamond facet is possible due to the close lattice matching and fcc symmetry of the (100) diamond facet. It is possible to grow more than a monolayer of γ-iron because the critical thickness of a strained multilayer is greater than a monolayer.[12] The determined critical thickness agrees well with the theoretical prediction.[12]
Thermo-optical radiation
During heat treatment, the smith causes phase changes in the iron-carbon system to control the mechanical properties of the material, often using annealing, quenching and tempering processes. In this context, the color of light, or "black body radiation," emitted by the workpiece is an approximate temperature sensor. Temperature is often measured by observing the color temperature of the work, with a transition from dark cherry red to orange-red (815 °C (1499 °F) to 871 °C (1600 °F)), corresponding to the formation of austenite in the middle and high carbon steel. In the visible spectrum, the brightness of this glow increases with temperature, and when it becomes cherry red, its intensity is near its lowest and may not be visible in ambient light. Therefore, blacksmiths typically austenitize steel under low light conditions to accurately determine the color of the glow.
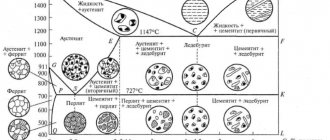
Characteristics of phase and structural components of iron-carbon alloys
In accordance with the previously given definitions of the phase and structural components of the system, in the iron-carbon system the phase components include: liquid solution (L), solid solutions: ferrite (α), austenite (γ), high-temperature ferrite (δ), as well as cementite and graphite (G).
The liquid solution in the iron-carbon system is a solution of carbon in molten iron. At temperatures significantly above the liquidus line (mainly above 1700°C), the liquid is a statistically disordered solution with a statistically dense packing. With slight overheating above the liquidus line, the liquid solution has a relatively regular structure. The liquid solution formed during the melting of δ-ferrite (up to 0.51% carbon) maintains short-range order in the bcc lattice of δ-iron. The liquid solution formed during the melting of austenite has a short-range order corresponding to the fcc lattice of γ-iron.
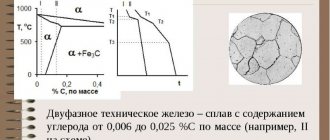
Ferrite is a solid solution of interstitial carbon in α-iron. The ferrite lattice is a body-centered cube with the arrangement of carbon atoms in relatively small octahedral voids of the lattice, which greatly distorts it. The solubility of carbon in ferrite is low.
At a temperature of 727? C, 0.02% C dissolves in ferrite; as the temperature decreases, the solubility decreases, reaching a value of 0.006% C at room temperature. The ferrite structure consists of relatively equiaxed polyhedral crystals separated from each other by thin high-angle boundaries. The structure of ferrite is usually revealed by etching with nitric acid solutions.
Ferrite up to the Curie point temperature (770? C) is highly ferromagnetic and conducts heat and electric current well. In the equilibrium state, ferrite is plastic (relative elongation of the order of 40%), has low strength and hardness (HB = 65 - I30, depending on the grain size).
Ferrite, depending on the nature of the ongoing phase transformations, in the structure of iron-carbon alloys can be in the form of various structural states: ferrite, as the basis of the alloy structure (F); ferrite, as the second (excess) phase, located along the boundaries of pearlite colonies, in the form of separate equiaxed or needle-shaped inclusions; ferrite, which is included as a phase in the composition of another structural component - pearlite or ferrite-graphite eutectoid.
At temperatures above the critical point A4, the modification of high-temperature δ-ferrite , which, like low-temperature α-ferrite, has a body-centered cubic lattice, but with larger parameters in comparison with it. δ-ferrite is paramagnetic.
Austenite is a solid solution of interstitial carbon in γ-iron. The austenite lattice is a face-centered cube (FCC). The carbon atoms are located in large octahedral lattice voids.
The solubility of carbon in austenite is significantly higher than in ferrite: 2.03 and 2.14% at eutectic transformation temperatures, respectively, in a stable and metastable system. As the temperature decreases, the solubility decreases to 0.69 and 0.80% in the mentioned systems, which corresponds to the temperatures of the eutectoid transformation in both systems.
Austenite is revealed in the structure in the same way as ferrite in the form of relatively equiaxed polyhedra, but differs from it in a significant number of twins in the body of the grain. Austenite is a paramagnetic component throughout the entire temperature range of its existence. Austenite is soft, although harder than ferrite (HB = 200-250). It is plastic (relative elongation 40-50% and above). The transformation of ferrite and ferrite-cementite mixture into austenite is accompanied by a decrease in volume.
The structural state of austenite (A) in iron-carbon alloys is similar to ferrite: it can be the only structural component in the alloy; form the basis of the alloy; enter into it as retained austenite; be contained as a phase component in the composition of a more complex structural component - a eutectic austenite-cementite mixture (ledeburite), which exists at temperatures above the eutectoid line on the iron-carbon diagram.
Cementite is a metastable compound of iron and carbon, corresponding to the formula Fe3C. Cementite has a complex orthorhombic lattice (Figure 4.6), the basis of which is a trihedral, slightly distorted prism formed by six iron atoms. Some iron atoms have 11 neighboring iron atoms, and some have 12. The voids are filled with carbon atoms. In this case, the structure of cementite is close in its structure to the structure of austenite, as well as to the densest hexagonal modification of ε - iron.
Cementite is a compound of almost constant composition. The solubility of iron in cementite occurs, but its value is very small and practically insignificant. With increasing temperature, cementite relatively easily decomposes into iron (austenite or ferrite) and graphite. This property of cementite underlies the phenomenon of graphitization, and is used to produce gray and ductile cast irons. Cementite is brittle, very hard (HB about 800), weakly magnetic up to a temperature of 210? C. Above this temperature, cementite is paramagnetic.
The structural state of cementite is determined mainly by the type of transformation during which it is formed. There is primary cementite (CI), which is large needle-shaped crystals formed during crystallization directly from liquid in hypereutectic white cast iron. Secondary cementite (CII) is released in hypereutectoid steels and hypoeutectic cast irons, mainly in the form of a network along the boundaries of austenite grains, and also in some cases in the form of coagulated particles or needles uniformly distributed throughout the volume of the austenite grain. Secondary cementite is an excess phase in iron-carbon alloys that is released from austenite during cooling as a result of a decrease in the solubility of carbon in austenite with decreasing temperature.
The precipitation of tertiary cementite (CIII) is typical for industrial iron and low-carbon steel. Tertiary cementite is released from ferrite as a result of a decrease in the solubility of carbon in ferrite with a decrease in temperature from 727? C to room temperature. Tertiary cementite in the structure of iron and low-carbon steel in the microstructure is observed in the form of thin veins along the boundaries of ferrite grains. Such precipitation of tertiary cementite embrittles iron and low-carbon steels. Therefore, such alloys are subjected to heat treatment in order to change the structural state of tertiary cementite. Its desired position in the structure of the alloy is uniformly dispersed precipitation in the volume of ferrite grains. This is achieved through hardening and aging.
In addition, cementite as a phase component is included in the complex two-phase structural components in iron-carbon alloys - pearlite and ledeburite . In this case, such cementite is called eutectoid and eutectic (Tse), respectively.
Graphite is the most important phase and structural component (G) of gray, malleable and high-strength cast irons, which determines their low shrinkage during crystallization, high anti-friction properties, low wear, high internal friction, which reduces vibration, and a number of other useful properties. Graphite is a hexagonal modification of carbon. At normal pressure, graphite is a stable component up to temperatures of about 4000? C.
In the graphite lattice, atoms are arranged in layers with hexagonal symmetry (Figure 4.7). In the first and third layers, the atoms are located on top of each other. In the second (middle) layer, the atoms are shifted along the largest diagonal of the hexagon by the value of the lattice parameter (the length of the side of the hexagon). The distance between the layers (3.35 kX) is significantly greater than the distances between neighboring atoms in a hexagonal layer. Due to the easy mobility of weakly bonded hexagonal layers, graphite is the least strong phase of iron-carbon alloys.
Graphite in the structure of iron-carbon alloys is either in the form of an excess phase (in hypereutectic gray cast iron) or as a phase component included in the austenite-graphite eutectic. Graphite has the form of branched crab-shaped inclusions. Eutectic graphite differs from primary graphite in its smaller size and greater branching.
After modifying liquid cast iron with magnesium and some other elements, as well as after annealing white cast iron into malleable iron, globular (flaky or spheroidal) graphite can be observed in the structure. This form of graphite provides increased strength and ductility of cast iron.
All the described phase components can simultaneously be structural components if they are in the form of excess phases in the alloy structure or form the basis of the alloy structure.
In addition to single-phase structural components, iron-carbon alloys also contain complex two-phase ones: pearlite, ledeburite, graphite-austenitic eutectic and ferrite-graphite eutectoid.
Pearlite is a eutectoid physicochemical mixture of two phases: ferrite and cementite, formed in a metastable iron-carbon system due to the diffusion separation of austenite according to the eutectoid reaction. Pearlite is formed when austenite is supercooled below the PSK line of the iron-carbon diagram. The structure of perlite is determined by the amount of supercooling at which decomposition occurs.
At low supercooling (20-30°C below the eutectoid transformation line), granular pearlite . Granular pearlite is a ferrite-cementite structure in which the base is ferrite, and granular, close to spherical, cementite inclusions are statistically evenly distributed throughout its volume.
With greater supercooling, a structure of lamellar pearlite , consisting of regularly alternating plates of cementite and ferrite, and the ferrite plates are approximately 7 times thicker than the cementite plates.
The absolute values of the thickness of cementite and ferrite plates, the distance between the same plates in the eutectoid mixture, called interplate distance , and characterizing the degree of dispersion of the structure, are determined by the degree of overcooling of austenite below the equilibrium temperature of the eutectoid reaction. The greater the degree of supercooling, the higher the dispersion of the ferrite-cementite eutectoid mixture. Highly dispersed ferrite-cementite mixtures are called sorbitol and troostite. Troostite is the most dispersed ferrite-cementite mixture.
Pearlite is present in the structure of steels and cast irons. The amount of pearlite increases in hypoeutectoid steels with increasing carbon content from 0.02 to 0.8%. Eutectoid steel has a purely pearlitic structure (100% pearlite).
A further increase in the carbon content in steel, corresponding to the transition to hypereutectoid steels, and then to cast irons, is accompanied by a decrease in the proportion of pearlite in the structure due to the appearance and increase in the amount of secondary, eutectic and, finally, primary cementite.
Pearlite in low-carbon steels appears first in the form of separate inclusions between ferrite grains, then, as its quantity increases, it gradually occupies an increasingly larger field of view in the structure on the surface of the section. While there is little pearlite in the structure, its structure is not revealed at low and medium magnifications of an optical microscope. In eutectoid and hypereutectoid steels, its lamellar structure is revealed even at low magnifications (×100 - 200).
In the structure of cast iron, pearlite is found both in the form of excess colonial structural components - products of the decomposition of excess austenite, and in the composition of ledeburite. The mechanical properties of pearlite are determined by its structural state. Calculation according to the rule of additivity of pearlite hardness, based on the known values of ferrite and cementite hardness, gives values of 150-180 HB. The experimentally determined hardness values of lamellar perlite, sorbitol and troostite are respectively 170 - 230, 230 - 330 and 330 - 400 HB. Thus, it can be seen that the higher the degree of dispersion of the ferrite-cementite mixture, the higher its hardness.
Ledeburite is a eutectic physicochemical mixture of austenite and cementite, formed as a result of eutectic crystallization from a liquid containing 4.3% carbon.
Ledeburite is a colonial structure, the basis of which is made up of cementite plates overgrown with branched austenite crystals. Austenite branches in the composition of ledeburite are located regularly throughout the entire volume of the eutectic cementite plate and have the form of rods of approximately cylindrical configuration. On a thin section, a colony of ledeburite, depending on the direction of the surface of the thin section relative to the austenitic branches, can look either in the form of a “granular” mixture in the cross section of the colony, or “platelike” in the longitudinal section. When the colony is sectioned at an angle to the plane of the cementite base, the sections of the austenite branches in the composition of ledeburite are of an elliptical configuration.
In addition to colonial (honeycomb) ledeburite, a eutectic mixture of austenite and cementite can occur in the form of lamellar eutectic, which is a package of thin cementite plates separated by austenite. Such packets are formed by two intertwined crystals of cementite and austenite. The probability of formation of lamellar ledeburite increases with increasing degree of supercooling of the liquid during crystallization. At the same time, the proportion of lamellar ledeburite in the structure of white cast iron increases. Most often, a packet of lamellar ledeburite forms the basis on which a colony of honeycomb ledeburite nucleates and grows.
At very high cooling rates, all ledeburite may turn out to be platelike. In this case, cementite branches, taking on the appearance of fan-shaped colonies. At even higher cooling rates, spherulite colonies appear. Ledeburite, consisting of a eutectic mixture of austenite and cementite, is stable in the temperature range from the eutectic to the eutectoid line on the iron-carbon diagram. When the temperature drops below 727°C, the austenite in ledeburite undergoes a eutectoid transformation, as a result of which at room temperature ledeburite is a eutectic mixture of pearlite and cementite. The structure of pearlite in ledeburite is the same as in alloys with lower carbon content (steels).
Ledeburite, like the cementite that forms its base, is hard, wear-resistant and has practically zero ductility. These properties of ledeburite underlie the use of this structure in white cast irons, which are used as one of the most wear-resistant materials.
Austenitic-graphite eutectic is formed in a stable iron-carbon system and is a mixture of graphite crystals, formed during the simultaneous release of both phase components from a liquid with a composition of 4.25% carbon. At low degrees of supercooling, eutectic graphite has, like primary graphite, a branched lamellar shape. An increase in the cooling rate leads to splitting of the graphite plates and the formation of spherical crystals. The eutectic austenite-graphite structure differs little from the separation of primary graphite crystals. The main difference between these structures is the size of the graphite inclusions. They are smaller in the eutectic than the primary crystals.
Ferrite-graphite eutectoid is a product of eutectoid decomposition of austenite containing 0.69% carbon, which is realized under very slow cooling conditions at temperatures below 738? C.
Ferrite-graphite eutectoid is a dispersed mixture of ferrite, which forms the basis of the alloy structure, and dispersed branched or spherical graphite particles, statistically evenly distributed in the ferrite. However, in most cases, eutectoid graphite, during the decomposition of austenite, is deposited on previously formed primary and eutectic graphite crystals. The eutectoid transformation with the formation of a ferrite-graft eutectoid is used in the heat treatment of cast iron and graphitized steel to obtain a ferrite-graphite structure with good antifriction properties while maintaining a sufficiently high ductility of the alloys.
In accordance with the previously given definitions of the phase and structural components of the system, in the iron-carbon system the phase components include: liquid solution (L), solid solutions: ferrite (α), austenite (γ), high-temperature ferrite (δ), as well as cementite and graphite (G).
The liquid solution in the iron-carbon system is a solution of carbon in molten iron. At temperatures significantly above the liquidus line (mainly above 1700°C), the liquid is a statistically disordered solution with a statistically dense packing. With slight overheating above the liquidus line, the liquid solution has a relatively regular structure. The liquid solution formed during the melting of δ-ferrite (up to 0.51% carbon) maintains short-range order in the bcc lattice of δ-iron. The liquid solution formed during the melting of austenite has a short-range order corresponding to the fcc lattice of γ-iron.
Ferrite is a solid solution of interstitial carbon in α-iron. The ferrite lattice is a body-centered cube with the arrangement of carbon atoms in relatively small octahedral voids of the lattice, which greatly distorts it. The solubility of carbon in ferrite is low.
At a temperature of 727? C, 0.02% C dissolves in ferrite; as the temperature decreases, the solubility decreases, reaching a value of 0.006% C at room temperature. The ferrite structure consists of relatively equiaxed polyhedral crystals separated from each other by thin high-angle boundaries. The structure of ferrite is usually revealed by etching with nitric acid solutions.
Ferrite up to the Curie point temperature (770? C) is highly ferromagnetic and conducts heat and electric current well. In the equilibrium state, ferrite is plastic (relative elongation of the order of 40%), has low strength and hardness (HB = 65 - I30, depending on the grain size).
Ferrite, depending on the nature of the ongoing phase transformations, in the structure of iron-carbon alloys can be in the form of various structural states: ferrite, as the basis of the alloy structure (F); ferrite, as the second (excess) phase, located along the boundaries of pearlite colonies, in the form of separate equiaxed or needle-shaped inclusions; ferrite, which is included as a phase in the composition of another structural component - pearlite or ferrite-graphite eutectoid.
At temperatures above the critical point A4, the modification of high-temperature δ-ferrite , which, like low-temperature α-ferrite, has a body-centered cubic lattice, but with larger parameters in comparison with it. δ-ferrite is paramagnetic.
Austenite is a solid solution of interstitial carbon in γ-iron. The austenite lattice is a face-centered cube (FCC). The carbon atoms are located in large octahedral lattice voids.
The solubility of carbon in austenite is significantly higher than in ferrite: 2.03 and 2.14% at eutectic transformation temperatures, respectively, in a stable and metastable system. As the temperature decreases, the solubility decreases to 0.69 and 0.80% in the mentioned systems, which corresponds to the temperatures of the eutectoid transformation in both systems.
Austenite is revealed in the structure in the same way as ferrite in the form of relatively equiaxed polyhedra, but differs from it in a significant number of twins in the body of the grain. Austenite is a paramagnetic component throughout the entire temperature range of its existence. Austenite is soft, although harder than ferrite (HB = 200-250). It is plastic (relative elongation 40-50% and above). The transformation of ferrite and ferrite-cementite mixture into austenite is accompanied by a decrease in volume.
The structural state of austenite (A) in iron-carbon alloys is similar to ferrite: it can be the only structural component in the alloy; form the basis of the alloy; enter into it as retained austenite; be contained as a phase component in the composition of a more complex structural component - a eutectic austenite-cementite mixture (ledeburite), which exists at temperatures above the eutectoid line on the iron-carbon diagram.
Cementite is a metastable compound of iron and carbon, corresponding to the formula Fe3C. Cementite has a complex orthorhombic lattice (Figure 4.6), the basis of which is a trihedral, slightly distorted prism formed by six iron atoms. Some iron atoms have 11 neighboring iron atoms, and some have 12. The voids are filled with carbon atoms. In this case, the structure of cementite is close in its structure to the structure of austenite, as well as to the densest hexagonal modification of ε - iron.
Cementite is a compound of almost constant composition. The solubility of iron in cementite occurs, but its value is very small and practically insignificant. With increasing temperature, cementite relatively easily decomposes into iron (austenite or ferrite) and graphite. This property of cementite underlies the phenomenon of graphitization, and is used to produce gray and ductile cast irons. Cementite is brittle, very hard (HB about 800), weakly magnetic up to a temperature of 210? C. Above this temperature, cementite is paramagnetic.
The structural state of cementite is determined mainly by the type of transformation during which it is formed. There is primary cementite (CI), which is large needle-shaped crystals formed during crystallization directly from liquid in hypereutectic white cast iron. Secondary cementite (CII) is released in hypereutectoid steels and hypoeutectic cast irons, mainly in the form of a network along the boundaries of austenite grains, and also in some cases in the form of coagulated particles or needles uniformly distributed throughout the volume of the austenite grain. Secondary cementite is an excess phase in iron-carbon alloys that is released from austenite during cooling as a result of a decrease in the solubility of carbon in austenite with decreasing temperature.
The precipitation of tertiary cementite (CIII) is typical for industrial iron and low-carbon steel. Tertiary cementite is released from ferrite as a result of a decrease in the solubility of carbon in ferrite with a decrease in temperature from 727? C to room temperature. Tertiary cementite in the structure of iron and low-carbon steel in the microstructure is observed in the form of thin veins along the boundaries of ferrite grains. Such precipitation of tertiary cementite embrittles iron and low-carbon steels. Therefore, such alloys are subjected to heat treatment in order to change the structural state of tertiary cementite. Its desired position in the structure of the alloy is uniformly dispersed precipitation in the volume of ferrite grains. This is achieved through hardening and aging.
In addition, cementite as a phase component is included in the complex two-phase structural components in iron-carbon alloys - pearlite and ledeburite . In this case, such cementite is called eutectoid and eutectic (Tse), respectively.
Graphite is the most important phase and structural component (G) of gray, malleable and high-strength cast irons, which determines their low shrinkage during crystallization, high anti-friction properties, low wear, high internal friction, which reduces vibration, and a number of other useful properties. Graphite is a hexagonal modification of carbon. At normal pressure, graphite is a stable component up to temperatures of about 4000? C.
In the graphite lattice, atoms are arranged in layers with hexagonal symmetry (Figure 4.7). In the first and third layers, the atoms are located on top of each other. In the second (middle) layer, the atoms are shifted along the largest diagonal of the hexagon by the value of the lattice parameter (the length of the side of the hexagon). The distance between the layers (3.35 kX) is significantly greater than the distances between neighboring atoms in a hexagonal layer. Due to the easy mobility of weakly bonded hexagonal layers, graphite is the least strong phase of iron-carbon alloys.
Graphite in the structure of iron-carbon alloys is either in the form of an excess phase (in hypereutectic gray cast iron) or as a phase component included in the austenite-graphite eutectic. Graphite has the form of branched crab-shaped inclusions. Eutectic graphite differs from primary graphite in its smaller size and greater branching.
After modifying liquid cast iron with magnesium and some other elements, as well as after annealing white cast iron into malleable iron, globular (flaky or spheroidal) graphite can be observed in the structure. This form of graphite provides increased strength and ductility of cast iron.
All the described phase components can simultaneously be structural components if they are in the form of excess phases in the alloy structure or form the basis of the alloy structure.
In addition to single-phase structural components, iron-carbon alloys also contain complex two-phase ones: pearlite, ledeburite, graphite-austenitic eutectic and ferrite-graphite eutectoid.
Pearlite is a eutectoid physicochemical mixture of two phases: ferrite and cementite, formed in a metastable iron-carbon system due to the diffusion separation of austenite according to the eutectoid reaction. Pearlite is formed when austenite is supercooled below the PSK line of the iron-carbon diagram. The structure of perlite is determined by the amount of supercooling at which decomposition occurs.
At low supercooling (20-30°C below the eutectoid transformation line), granular pearlite . Granular pearlite is a ferrite-cementite structure in which the base is ferrite, and granular, close to spherical, cementite inclusions are statistically evenly distributed throughout its volume.
With greater supercooling, a structure of lamellar pearlite , consisting of regularly alternating plates of cementite and ferrite, and the ferrite plates are approximately 7 times thicker than the cementite plates.
The absolute values of the thickness of cementite and ferrite plates, the distance between the same plates in the eutectoid mixture, called interplate distance , and characterizing the degree of dispersion of the structure, are determined by the degree of overcooling of austenite below the equilibrium temperature of the eutectoid reaction. The greater the degree of supercooling, the higher the dispersion of the ferrite-cementite eutectoid mixture. Highly dispersed ferrite-cementite mixtures are called sorbitol and troostite. Troostite is the most dispersed ferrite-cementite mixture.
Pearlite is present in the structure of steels and cast irons. The amount of pearlite increases in hypoeutectoid steels with increasing carbon content from 0.02 to 0.8%. Eutectoid steel has a purely pearlitic structure (100% pearlite).
A further increase in the carbon content in steel, corresponding to the transition to hypereutectoid steels, and then to cast irons, is accompanied by a decrease in the proportion of pearlite in the structure due to the appearance and increase in the amount of secondary, eutectic and, finally, primary cementite.
Pearlite in low-carbon steels appears first in the form of separate inclusions between ferrite grains, then, as its quantity increases, it gradually occupies an increasingly larger field of view in the structure on the surface of the section. While there is little pearlite in the structure, its structure is not revealed at low and medium magnifications of an optical microscope. In eutectoid and hypereutectoid steels, its lamellar structure is revealed even at low magnifications (×100 - 200).
In the structure of cast iron, pearlite is found both in the form of excess colonial structural components - products of the decomposition of excess austenite, and in the composition of ledeburite. The mechanical properties of pearlite are determined by its structural state. Calculation according to the rule of additivity of pearlite hardness, based on the known values of ferrite and cementite hardness, gives values of 150-180 HB. The experimentally determined hardness values of lamellar perlite, sorbitol and troostite are respectively 170 - 230, 230 - 330 and 330 - 400 HB. Thus, it can be seen that the higher the degree of dispersion of the ferrite-cementite mixture, the higher its hardness.
Ledeburite is a eutectic physicochemical mixture of austenite and cementite, formed as a result of eutectic crystallization from a liquid containing 4.3% carbon.
Ledeburite is a colonial structure, the basis of which is made up of cementite plates overgrown with branched austenite crystals. Austenite branches in the composition of ledeburite are located regularly throughout the entire volume of the eutectic cementite plate and have the form of rods of approximately cylindrical configuration. On a thin section, a colony of ledeburite, depending on the direction of the surface of the thin section relative to the austenitic branches, can look either in the form of a “granular” mixture in the cross section of the colony, or “platelike” in the longitudinal section. When the colony is sectioned at an angle to the plane of the cementite base, the sections of the austenite branches in the composition of ledeburite are of an elliptical configuration.
In addition to colonial (honeycomb) ledeburite, a eutectic mixture of austenite and cementite can occur in the form of lamellar eutectic, which is a package of thin cementite plates separated by austenite. Such packets are formed by two intertwined crystals of cementite and austenite. The probability of formation of lamellar ledeburite increases with increasing degree of supercooling of the liquid during crystallization. At the same time, the proportion of lamellar ledeburite in the structure of white cast iron increases. Most often, a packet of lamellar ledeburite forms the basis on which a colony of honeycomb ledeburite nucleates and grows.
At very high cooling rates, all ledeburite may turn out to be platelike. In this case, cementite branches, taking on the appearance of fan-shaped colonies. At even higher cooling rates, spherulite colonies appear. Ledeburite, consisting of a eutectic mixture of austenite and cementite, is stable in the temperature range from the eutectic to the eutectoid line on the iron-carbon diagram. When the temperature drops below 727°C, the austenite in ledeburite undergoes a eutectoid transformation, as a result of which at room temperature ledeburite is a eutectic mixture of pearlite and cementite. The structure of pearlite in ledeburite is the same as in alloys with lower carbon content (steels).
Ledeburite, like the cementite that forms its base, is hard, wear-resistant and has practically zero ductility. These properties of ledeburite underlie the use of this structure in white cast irons, which are used as one of the most wear-resistant materials.
Austenitic-graphite eutectic is formed in a stable iron-carbon system and is a mixture of graphite crystals, formed during the simultaneous release of both phase components from a liquid with a composition of 4.25% carbon. At low degrees of supercooling, eutectic graphite has, like primary graphite, a branched lamellar shape. An increase in the cooling rate leads to splitting of the graphite plates and the formation of spherical crystals. The eutectic austenite-graphite structure differs little from the separation of primary graphite crystals. The main difference between these structures is the size of the graphite inclusions. They are smaller in the eutectic than the primary crystals.
Ferrite-graphite eutectoid is a product of eutectoid decomposition of austenite containing 0.69% carbon, which is realized under very slow cooling conditions at temperatures below 738? C.
Ferrite-graphite eutectoid is a dispersed mixture of ferrite, which forms the basis of the alloy structure, and dispersed branched or spherical graphite particles, statistically evenly distributed in the ferrite. However, in most cases, eutectoid graphite, during the decomposition of austenite, is deposited on previously formed primary and eutectic graphite crystals. The eutectoid transformation with the formation of a ferrite-graft eutectoid is used in the heat treatment of cast iron and graphitized steel to obtain a ferrite-graphite structure with good antifriction properties while maintaining a sufficiently high ductility of the alloys.