Cast iron consists of carbon, iron and some impurities. It is one of the main materials of ferrous metallurgy. Cast iron is used in the manufacture of household and utility items, machine parts and in other industries. It is used in production, focusing and taking into account its properties and characteristics.
This article is precisely intended to tell you about the density of high-strength, liquid, white and gray cast iron, its melting points and specific heat capacity will also be considered separately.
Thermal properties of cast iron
Cast iron, like any metal, has the following properties: thermal, physical, mechanical, hydrodynamic, electrical, technological, chemical. Let's look at each properties in more detail.
This video talks about the structure and composition of cast iron alloys and the dependence of their properties on a specific composition:
Heat capacity
The thermal capacity of cast iron is determined using the displacement rule. When the heat capacity of cast iron reaches a temperature period, the beginning of which begins at a temperature whose value is greater than phase transformations and ends at a level equal to the melting temperature, then the heat capacity of cast iron takes on a value of 0.18 cal/Ho C.
If the value of the melting temperature exceeds the absolute value, then the heat capacity is equal to 0.23 ± 0.03 cal/Ho C. If the solidification process occurs, then the thermal effect is equal to 55 ± 5 cal. The thermal effect depends on the amount of pearlite when pearlite transformation occurs. Typically it takes a value of 21.5 ± 1.5 cal/G.
The volumetric heat capacity is taken to be the product of the specific gravity and the specific heat capacity. For solid cast iron this value is 1 cal/cm3*ºС, for liquid cast iron – 1.5 cal/cm3*ºС.
The specific heat capacity of cast iron is 540 J/kg C.
Specific heat capacity of cast iron and other metals in table form
Thermal conductivity
Unlike heat capacity, thermal conductivity is not determined by the displacement rule. Only if the amount of graphitization changes, the composition of the cast iron will affect the thermal conductivity.
Thermal diffusivity
The thermal diffusivity value of solid cast iron (for large calculations) can be taken equal to its thermal conductivity, and that of liquid cast iron – 0.03 cm2*/sec.
Read below about the melting point of cast iron.
Melting temperature
Cast iron melts at a temperature of 1200ºС. This temperature value is 300 degrees lower than the melting point of steel. With an increased carbon content, this chemical element has a close connection at the molecular level with iron atoms.
During the process of melting cast iron and its crystallization, the carbon component cannot completely penetrate the structural lattice of iron. As a result, the material cast iron takes on the property of brittleness. Cast iron is used for parts that require increased strength. However, cast iron is not used in the manufacture of objects that will be subject to constant dynamic loads.
The table below shows the melting point of cast iron in comparison with other metals.
Melting point of cast iron and other metals
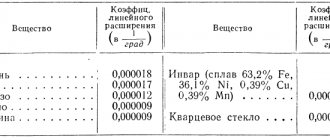
Cast iron density, melting point and linear expansion coefficient
The table shows the density of cast iron of various grades, as well as the melting point of cast iron and its coefficient of thermal linear expansion (CTLP).
It should be noted that the density of cast iron, depending on the grade, ranges from 6600 to 7700 kg/m3 .
The melting point of cast iron ranges from 1095 to 1315°C, and its KTLR ranges from 10.5 to 18·10-6 1/deg. Cast iron density, melting point and expansion coefficient
| Density of cast iron, kg/m3 | |
| Gray cast iron is the least dense high carbon | 6600-6950 |
| Regular gray cast iron medium density | 7000-7300 |
| High quality low carbon cast iron | 7400-7500 |
| Heat resistant, heat resistant | 7500-7600 |
| High-alloy cast iron, austenitic class | 7500-7700 |
| Melting temperature of cast iron, °C | |
| Regular gray cast iron | 1095-1315 |
| Heat-resistant cast iron | 1300 |
| Linear expansion coefficient of cast iron (KTLR), 1/deg | |
| Regular gray at temperatures 20…450°C | 10,5·10-6 |
| Regular gray at temperatures 20...750°C | 14·10-6 |
| Highly alloyed austenitic class at temperatures 20…150°C | (16…18)·10-6 |
| Heat-resistant cast iron at temperatures 20…250°C | 16,7·10-6 |
| Heat-resistant cast iron at temperatures 250…750°C | 17,6·10-6 |
Sources:
- Kazantsev E.I. Industrial furnaces. Reference manual for calculations and design.
- Chirkin V.S. Thermophysical properties of nuclear technology materials. Directory.
physical characteristics
Weight
The weight of the material varies depending on the amount of fixed carbon and the presence of a certain percentage of porosity. The specific gravity of cast iron at the melting point can be significantly reduced depending on the presence of impurities in the cast iron.
In addition, the linear expansion of the metal and the structure of cast iron changes depending on the state of each indicator. That is, these are dependent quantities.
The specific gravity of each cast iron differs depending on the type of material. Gray cast iron has a specific gravity of 7.1±0.2 g/cm3, white cast iron has a specific gravity of 7.5±0.2 g/cm3, and malleable cast iron has a specific gravity of 7.3±0.2 g/cm3.
The video below will tell you about some of the physical properties of cast iron:
Volume
The volume of cast iron, passing through the temperature of phase transformations, reaches an increase of 30%. However, when heated to 500ºC, the volume increases by 3%. Growth is aided by graphite-forming elements. Carbide-forming components inhibit volume growth. The same growth is prevented by applying galvanic coatings to the surface.
The carbon content is usually at least 2.14%. Thanks to the carbon content, cast iron has excellent hardness. However, the plasticity and malleability of the material suffers against this background.
We will talk about the density of cast iron below.
Density
The density of the described material, cast iron, is 7.2 g/cm3. If we compare other metals and alloys with cast iron, this density value is quite high.
Due to its good density, cast iron is widely used for casting various parts in industry. In terms of this property, cast iron is only slightly inferior to some steels.
Thermal conductivity of cast iron
The table shows the thermal conductivity values of cast iron depending on temperature and composition. The thermal conductivity of liquid cast iron at a temperature of 1400°C is also indicated.
Thermal conductivity values are presented for the following grades of cast iron: ordinary cast iron, molybdenum-chromium cast iron, molybdenum, chromium-nickel, manganese-nickel, nickel-resist cast iron, nickel-resist, nickel-resist, chrome-aluminum, cuprous, ordinary pure, gray cast iron, annealed malleable cast iron, liquid cast iron.
The thermal conductivity of cast iron is given depending on temperature in the range from 0 to 400°C. According to the table, it can be seen that with increasing temperature, the thermal conductivity of cast iron decreases . The thermal conductivity values of cast iron of common grades are also indicated in this table.
Mechanical Features
Tensile strength
The compressive strength of cast iron depends on the structure of the material itself. The components of the structure gain their strength along with an increase in the level of dispersion. The tensile strength is strongly influenced by the number, size, distribution and formagraphite inclusions. The tensile strength decreases by a noticeable amount if the graphite inclusions are arranged in the form of a chain. This arrangement reduces the cohesion of the metal mass.
The tensile strength reaches its maximum value when the graphite takes on a spheroidal shape. This form is obtained without the influence of temperature, but when cerium and magnesium are included in the cast iron mass.
- When the melting temperature increases to 400ºС, the tensile strength does not change.
- If the temperature rises above this value, the tensile strength decreases.
- Note that at temperatures from 100 to 200ºС, the tensile strength can decrease by 10-15%.
Plastic
The ductility of cast iron largely depends on the shape of the graphite, and also depends on the structure of the metal mass. If graphite inclusions have a spheroidal shape, then the percentage of elongation can reach 30.
- In ordinary gray cast iron, the elongation reaches only a tenth.
- In annealed gray cast iron, the elongation is 1.5%.
Elasticity
Elasticity depends on the shape of the graphite. If the graphite inclusions did not change, and the temperature increased, then the elasticity remains at the same value.
To an engineer about aluminum
The most attractive physical property of aluminum for engineers is its density of 2.7 g/cm3, which is only a third of the density of steel.
Corrosion resistance of aluminum
The second most important property is its good corrosion resistance, although aluminum is not a very noble metal from a chemical point of view.
This is because "fresh" aluminum (and aluminum alloys) reacts with oxygen and water vapor in the air to form a thin, dense oxide film that protects the underlying metal from further interaction with the environment.
Therefore, commercial aluminum and most of its alloys without copper alloying show very good corrosion resistance in liquids with a pH in the acid range from 5 to 8, which corresponds to most atmospheric environmental conditions.
Thermal expansion of aluminum
The linear thermal expansion of aluminum and its alloys is 24·10-6 per 1 degree Celsius - twice as much as that of steels.
This must be taken into account in many designs in which it is necessary to ensure free thermal expansion of elements.
When thermal expansion (or contraction) is limited in an aluminum element, due to the lower modulus of elasticity, stresses arise that are 2/3 of the stresses that would arise in a similar steel element.
Modulus of elasticity of aluminum
The elastic modulus of aluminum is 70,000 MPa, only a third of the elastic modulus of steel. This entails significant consequences for the geometry of the structure, since the deflections of the beams, the load-bearing capacity of the columns, i.e. their lateral buckling or local buckling directly depends on the elastic modulus.
Rigidity of aluminum profiles
In many building structures, a critical parameter of profiles is their rigidity.
If a steel profile is replaced with an aluminum one while maintaining its rigidity, then thickening all the walls three times is not entirely economical, since aluminum is exactly the same three times lighter than steel.
However, lightweighting structures through the use of aluminum is a natural desire, both for physical and economic reasons.
When designing beams, there is a practical and proven rule: increase all dimensions except the width by 1.4 times and you will get a cross section with a moment of inertia almost three times greater. Then for a profile with the same stiffness (E · I) you will save about 50% of the weight.
In this case, the loss of rigidity in relation to lateral buckling is compensated to some extent. Taking into account the fact that often standard steel profiles are not very optimal, you can save more than 50% of the weight. This can be clearly seen from Figure 1.
If there are no height restrictions and lateral buckling is not a design parameter, weight savings of up to 60% can be achieved.
If the rigidity of the element is not important, and the strength of the steel is close to the strength of the aluminum alloy, then savings can be up to 70%, but this is the final limit of possible weight savings.
Picture 1
These considerations lead to a second important point. If the moment of inertia of the profile increases three times with an increase in the height of the profile only by 1.4 times, then the moment of resistance of the section will increase accordingly by 3: 1.4 = 2.1 times.
Therefore, the stresses in an aluminum beam compared to a steel beam will be more than two times less.
Now it is clear why the designer does not need to immediately “grasp” high-strength aluminum alloys, and why less alloyed aluminum alloys 6060 and 6063 (AD31) are so popular.
Heating aluminum
Like other metals, the strength of aluminum decreases with increasing temperature. Up to some temperatures, this phenomenon is reversible, that is, after cooling, the material returns to the same properties as before heating.
Up to a temperature of about 80 °C, the drop in strength can be neglected for all alloys and states. Above 80°C, some design situations may require consideration of the creep effect.
Thermally strengthened alloys begin to lose strength at temperatures above 110 °C, and the extent of this phenomenon depends on the duration of heating.
Alloys that are not hardened by heat treatment begin to lose strength in cold-worked states at temperatures above 150 °C and also depending on the duration of heating. After heating non-thermally hardenable alloys in the annealed “O” state, no irreversible loss of strength occurs.
It is believed that short heating of thermally strengthened aluminum profiles to a temperature of 180-200 °C for 10-15 minutes, which occurs when powder paints are “melted,” does not lead to a serious loss of strength.
Welding of aluminum alloys
Much more serious is the loss of strength of aluminum alloys during welding. Here the temperature rises so high due to local melting that the drop in strength near the weld must be taken into account. Alloys that are not thermally hardenable lose all of their strength obtained during cold hardening and return to the annealed “O” state.
Heat-hardening aluminum alloys in the T6 condition lose approximately 40% of their strength (Figure 2) with the exception of alloy 7020, which loses only 20%. All these alloys do not reach the state of complete annealing, since a certain hardening effect is inevitable when the weld cools.
Requirements for the strength characteristics of the material in the weld zone are established and controlled based on the results of testing samples.
Figure 2
Hydrodynamic properties
Dynamic viscosity
Viscosity becomes less if the amount of manganese in cast iron increases. A decrease in viscosity was also noticed with a decrease in the content of sulfur impurities and other non-metallic components.
The process is affected by the temperature value. Thus, the viscosity becomes less when the ratio of two temperatures is directly proportional (the temperature of the experiment and the start of solidification).
Surface tension
This figure is 900±100 dynes/cm2. The value increases as the amount of carbon decreases and undergoes significant changes in the presence of non-metallic components.
Toxicity
Cookware is often made from cast iron. The fact is that cast iron as a material is non-toxic and tolerates temperature changes well.
Chemical properties
The corrosion resistance of a material depends on the external environment and its structure. If we consider cast iron from the side of decreasing electrode potential, then its components have the following arrangement: graphite-cementite, phosphide eutectic-ferrite.
It should be noted that the potential difference between graphite and ferrite is 0.56 V. If the dispersion increases, the corrosion resistance becomes less. With a strong decrease in dispersion, the opposite effect occurs, and corrosion resistance decreases. Alloying elements also affect the resistance of cast iron.