Oxygen cylinder pressure: tank characteristics
We will send the material by email
Safety precautions
The oxygen cylinder design is very simple, but reliable. The main thing is to accurately observe the parameters of the gas pumped inside so that the container does not rupture. During operation and storage, some very strict requirements must be observed.
Refilling with oxygen is a complex process, because it is the gas that is pumped into the welding cylinders. And it reaches the distribution station in a liquid state. Such oxygen is much safer than gaseous oxygen, but it evaporates quickly and in large quantities, which is not profitable financially. But manufacturers take such losses because safety comes first. Moreover, liquid oxygen is transported in large quantities (road and rail tanks). If such a volume catches fire and explodes, the losses will be several times greater.
Gas is pumped into cylinders using pumping and pumpless methods. In this case, filling does not occur with supercooled oxygen. When performing any actions with a cylinder, it is very important to comply with accuracy and safety requirements. The most vulnerable point is the valve; most often it is the valve that fails because it is subjected to repeated opening and closing.
It cannot be repaired, you can only replace it with a new one. Doing this with your own hands is prohibited; such an operation can only be carried out in a factory setting. Here it is important to follow the installation rules, which are based on pressing, that is, screwing in under a certain pressure. Then the cylinder itself with the valve is checked with test pressure. By the way, testing is hydraulic. Water is pumped inside the cylinder under a pressure of 225 or 300 kg/cm², which remains there for 5 minutes. After which the pressure is reduced to working pressure - 150 or 200 kg/cm².
It should be noted that the same technology is used to check the cylinders themselves to detect leaks. If nothing is found: all joints and walls have not become wet, then the test was successful and the device itself can be used further.
Source
Cylinder parameters
When oxygen oxidizes, it releases a lot of heat into the atmosphere. Excess energy can cause fire or detonation (explosion). Cylinders are a safe vessel that facilitates movement when using a substance. When choosing a container, pay attention to 3 parameters.
Structure
Structures for oxygen transportation are made using a seamless method from high-alloy or carbon steel grades. The thickness of the walls of the tanks is 6-8 mm. The cylinders are made in the shape of a cylinder with a rounding on one side. The container has a convex bottom. At the bottom there is a shoe made of metal tape, which helps keep the product in an upright position.
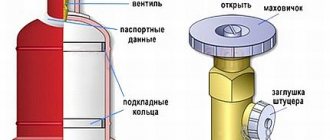
In the neck area there is a ring for mounting a safety cap. The device is installed on top of the valve. The element is used to protect against the ingress of explosive components and also protects the gearbox from mechanical damage.
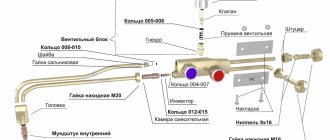
An important additional part of the oxygen cylinder is the valve. The device is made of brass. The alloy of copper and zinc is much superior to other metals in terms of chemical properties. The substance has high resistance to oxidation and corrosion processes, which is necessary when working with gas.
A stamped brass valve is a shut-off part, thanks to which the element is connected to an oxygen cylinder. At the bottom of the body there is a threaded shank for the neck, and on the side there is a fitting for the tube. A valve and a coupling with a seat are screwed between the container and the element, and a copper seal is installed between the components.
When the valve rotates clockwise, the mechanism closes the gas hole. During the reverse movement, the valve rises, opens the well and oxygen begins to escape. The reliability of the equipment is ensured by the mechanical structure of the structure.
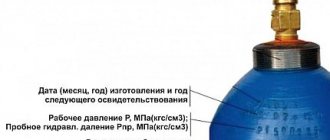
According to GOST, cylinders must be painted blue. The name of the gas is written across the tank in black paint. The manufacturer's mark and information about the container are stamped on the upper oval part of the surface:
To ensure that the data is clearly visible, the top is left unpainted. The weight of a standard cylinder varies from 67 to 105 kg. Weight of additional parts – up to 10 kg. The height of forty-liter models is 1.37-1.46 m, fifty-liter models are 1.68-1.76 m.
Pressure
The pressure characteristic is important when refilling oxygen cylinders. Professionals use a special formula that allows them to accurately determine the parameter. The calculations take into account the capacity of a particular model in cubic decimeters.
Types of oxygen
The choice of variety depends on the tasks that the substance must perform. Inexpensive technical raw materials are used for welding and cutting metal. The composition of oxygen must comply with the standards of GOST 5583-48.
The volatile component is produced by low-temperature rectification from air. The gas in the compressor is first compressed, then sharply cooled to room temperature. The result is concentrated liquid oxygen. The substance can be isolated by electrolysis of water.
Additionally, gas is divided into 2 grades, which differ in the proportion of different impurities. The composition of the technical form may contain minor inclusions of compounds and a subtle odor. The characteristics do not affect the operation, so the substance is not purified.
Medical grade is a concentrated version that contains no impurities or extraneous odors. Gas is supplied only in new cylinders, and the composition must fully comply with the standards of GOST 5583-48. Raw materials have a very complex, expensive and labor-intensive production process, which is reflected in the cost.
Medical liquefied oxygen is classified as a medicinal product, so the manufacturer must have a license. Raw materials go through several stages of testing, allowing defects to be detected at any stage. You can distinguish the type from the technical one by the inscription on the cylinder and by the accompanying documentation.
Oxygen: formula, density, mass, properties, methods of production and use
Gases: nitrogen, oxygen, hydrogen, carbon dioxide, argon
Application
oxygen
Gaseous oxygen today is produced on an industrial scale from atmospheric air. Its production is relevant and in demand today. There is special equipment that allows you to obtain oxygen in the required volumes.
In laboratory conditions, gaseous (and then liquid) oxygen is most often obtained using the electrolysis of aqueous solutions of alkalis. There is also another way to obtain a small amount of oxygen - the interaction of an acidified solution of hydrogen peroxide and a solution of potassium permanganate.
Gaseous oxygen is in demand today in many professional human activities.
Oxygen gas
Oxygen is one of the most important elements on the planet. It participates in the process of respiration, in the metabolism of living organisms, as well as in the circulation of substances in the biosphere. In addition, it promotes rotting and decomposition of organic matter.
Under normal conditions, it is a colorless gas that is tasteless and odorless. It is heavier than air and difficult to dissolve in water. Chemically, it is very active and is capable of forming compounds with almost all elements.
In a free state in the form of O2 molecules consisting of two oxygen atoms, it is found in the atmosphere. Due to this structure, the element is also called “dioxygen,” but it can exist in other variations. Under certain conditions, its atoms can form “trioxygen” with the O3 molecule, which is a blue gas called ozone with a specific odor.
In the atmosphere, the oxygen content is approximately 21% by mass, in the earth's crust its share is much higher and is about 47% by mass.
The element is part of more than one and a half thousand different rocks and minerals, most of which are silicates. There it is present in the form of compounds.
In water, its content reaches 85%, and this is not surprising, because oxygen atoms form water together with the element hydrogen.
History of discovery
It is officially believed that oxygen was discovered by the English chemist Joseph Priestley on August 1, 1774 by decomposing mercuric oxide in a hermetically sealed vessel (Priestley directed sunlight at this compound using a powerful lens). 2HgO →ot 2Hg + O2↑
However, Priestley initially did not realize that he had discovered a new simple substance; he believed that he had isolated one of the constituent parts of air (and called this gas “dephlogisticated air”). Priestley reported his discovery to the outstanding French chemist Antoine Lavoisier. In 1775, A. Lavoisier established that oxygen is a component of air, acids and is found in many substances.
A few years earlier (in 1771), oxygen was obtained by the Swedish chemist Karl Scheele. He calcined saltpeter with sulfuric acid and then decomposed the resulting nitric oxide.
Scheele called this gas “fire air” and described his discovery in a book published in 1777 (precisely because the book was published later than Priestley announced his discovery, the latter is considered the discoverer of oxygen). Scheele also reported his experience to Lavoisier.
An important step that contributed to the discovery of oxygen was the work of the French chemist Pierre Bayen, who published works on the oxidation of mercury and the subsequent decomposition of its oxide.
Finally, A. Lavoisier finally figured out the nature of the resulting gas, using information from Priestley and Scheele. His work was of enormous importance because thanks to it, the phlogiston theory, which was dominant at that time and hampered the development of chemistry, was overthrown.
Lavoisier conducted experiments on the combustion of various substances and disproved the theory of phlogiston, publishing results on the weight of the burned elements.
The weight of the ash exceeded the original weight of the element, which gave Lavoisier the right to claim that during combustion a chemical reaction (oxidation) of the substance occurs, and therefore the mass of the original substance increases, which refutes the theory of phlogiston.
Thus, the credit for the discovery of oxygen is actually shared between Priestley, Scheele and Lavoisier.
Liquid oxygen
Like other substances, oxygen can exist in various states of aggregation. To turn a gas into a solid or liquid, it must be very cool.
At a pressure of 51 atmospheres, it becomes liquid already at -119 °C. At normal pressure, the transformation occurs only at -183 °C.
When cooled to -220°C, it hardens to form light blue snow-like crystals.
In the liquid state, oxygen turns blue and enhances some of the properties of the gaseous substance. Thus, it behaves more aggressively in chemical reactions, and also becomes a strong paramagnetic and can be attracted by a magnet.
It boils only at -183 °C and melts at +219 °C. Due to its resistance to such low temperatures, liquid oxygen has cryogenic properties and can be used as a refrigerant.
Under normal conditions, it evaporates quickly, turning into gas. At the same time, it intensively absorbs heat and cools the surrounding air, causing a halo of fog to appear next to it. During evaporation, the volume of oxygen increases several hundred times.
Thus, 1 cm3 of liquid forms almost 800 cm3 of gas.
Scope of use
Compressed oxygen is a popular gas, the scope of which depends on the type of raw material. The medical substance is used during the resuscitation of patients. The element has a beneficial effect on the heart and lungs, so therapeutic procedures are often prescribed for health problems. The component is taken to saturate cocktails during oxygen starvation.
Technical gas heats up quickly and maintains high temperatures for a long time. The resulting continuous jet burns through metal of any density, which allows you to cut or solder parts. The characteristic is useful both in construction and in domestic use. In metallurgy, the substance increases the efficiency of furnaces, thereby improving the quality of the finished product.
History of the discovery of oxygen
The discovery of oxygen is credited to Joseph Priestley. He had a laboratory equipped with instruments for collecting gases. He tested its physiological effects on himself and on mice. Priestley found that after inhaling the gas, a pleasant lightness was felt for some time. Mice in a hermetically sealed jar of air suffocate faster than in a jar of O2. Since Priestley was an adherent of the phlogiston theory, he never found out what was in his hands. He only described this gas, without even realizing what he was describing. But the laurels of the discovery of oxygen belong to Antoine Laurent de Lavoisier, who gave it its name.
Lavoisier staged his famous experiment, which lasted 12 days. He heated mercury in a retort. When boiling, its red oxide was formed. When the retort was cooled, it turned out that the air in it had decreased by almost 1/6 of its volume, and the remainder of the mercury weighed less than before heating. But when the mercury oxide was decomposed by strong calcination, everything returned: both the lack of mercury and the “disappeared” oxygen.
Subsequently, Lavoisier established that this gas is part of nitric, sulfuric, and phosphoric acids. He mistakenly believed that O2 was necessarily part of acids, and therefore called it “oxygenium,” which means “acid-producing.” Nowadays, acids devoid of “oxygenium” are well known (for example: hydrochloric, hydrogen sulfide, hydrocyanic, etc.).
Terms of use
Oxygen is a dangerous gas that can explode upon contact with fire or oil. The sealed container protects the raw material from contact with unfavorable conditions, but trouble can arise if it falls or is heated by the sun's rays. There are recommendations to keep people safe when using volatile substances.
Welding
Oxygen is prohibited from combining with asphalt and coal, wood and paper. Materials saturated with concentrated compressed liquid can detonate. After working with the substance, you need to ventilate your clothes for half an hour.
Combustible and flammable components are located at least 5-10 m from the gas container. For safety reasons, the tank is placed in a vertical position. Before connecting, degrease the cylinders with a rag. The structure is connected firmly and steadily, otherwise the structure will collapse.
If the valve is frozen, it is forbidden to warm it up with fire. It is better to keep the tank in a warm room or use hot water. The cap is easier to remove with a key, but some models can be unscrewed by hand. When moving, try not to make sudden jerks, otherwise fire may occur.
When inspecting the surface of the locking part, pay attention to dents and scratches. It is prohibited to operate equipment with damaged containers or with expired expiration dates. If accidentally ingested, the ingredients can burn the mucous membranes of the eyes and freeze the skin. Work with the substance is carried out wearing protective gloves and a mask.
Refueling
Reliability and safety of operation of the tank depends on correct filling. The substance enters the station in liquid form and enters the cylinders in gas form. The procedure is carried out through a valve to which a pipe is hermetically connected. One end is connected to the base, the other to the container.
The connecting parts are screwed tightly, but not pinched. Be sure to ensure the tightness of the connection. The tap is carefully opened until a characteristic hissing sound occurs. The cessation of noise is a sign that the container is full.
Injection for large tanks is carried out using a pump; small vessels can be pumped without additional equipment. The pressure in a full oxygen cylinder is reduced by a reducer, otherwise the structure may break. Upon completion of the procedure, the valve is screwed in and the pipe is unscrewed.
How to store and move
The storage of gas tanks is prescribed in GOST 26460. The room must have electricity, heating and exhaust ventilation. It is prohibited to place other gases, flammable ingredients and heating devices near the substance. The building is located away from industrial buildings. The tanks are kept in metal boxes with holes, away from the direct sun.
To move oxygen cylinders, special equipment (stretchers, carts) is used. Containers are prohibited from being carried on the arms (shoulders). When transporting over long distances, you need a car with a cargo compartment. The tanks are laid horizontally in cells; felt is used for compaction and protection against collisions. If events take place in the heat, the structure is hidden under a tarpaulin.
The empty container is marked with chalk with the word “Empty” and the cap and plug are closed. Oxygen from the cylinders cannot be completely consumed, so a little substance is left under a pressure of 0.5 kg/cm2. The residues are needed for laboratory analysis of gas composition at a gas station. If the information matches the data from the previous procedure, then there is no need to flush the equipment.
Equipment
The main additional element of the oxygen tank is the valve. It is made from brass. A protective cap must be installed over the valve; it can be aluminum or plastic. Usually the cap comes as an integral part. But they are often lost, so the protective device can be made from any material with your own hands. Reliability and tightness are important here. The valve is screwed into the cylinder itself using a conical thread
The second most important element is the shoe. It is on him that the entire weight load falls. It is made from steel strip, which is shaped into a square cross-section. GOST does not define exactly how it should be fixed to the cylinder, so some manufacturers weld it, others press it in.
Oxygen in cylinders
You can buy oxygen in cylinders of 40 liters and on request 5, 10 and 20 liters, fill the cylinders with oxygen, and also buy liquid oxygen.
Oxygen is the most popular technical gas that is required in many industries including metallurgy, food industry, medicine, cosmetology and many others. It is necessary for oxidative processes and without it the combustion process is impossible.
This gas, heavier than air, has neither color nor odor. It is also very important that it is non-toxic, which means it is safe for humans and the environment. However, high concentrations of oxygen cause some chemical materials to ignite.
Requires a high production culture.
Oxygen – producing acids
Content
Oxygen under normal conditions (temperature and pressure) is a transparent gas without odor, taste or color.
It is not a flammable gas, but can actively support combustion. In terms of chemical activity among non-metals, it ranks second after fluorine.
All elements, except noble metals (platinum, gold, silver, rhodium, palladium, etc.) and inert gases (helium, argon, xenon, krypton and neon), undergo oxidation reactions and form oxides. The oxidation process of elements, as a rule, is exothermic (with the release of heat) in nature. It is also necessary to take into account the fact that with increasing temperature, pressure or the use of catalysts, the rate of the oxidation reaction increases sharply.
OIL HAZARDOUS.
Storage and transportation of oxygen.
Packaging and storage of oxygen is carried out in accordance with the requirements of GOST 26460. All types of transport, including pipelines, are used to transport technical and medical oxygen. Transportation and storage of gaseous oxygen is carried out in metal oxygen cylinders manufactured in accordance with GOST 949-73. Oxygen cylinders are blue in color and have the word “oxygen” written in white. At an ambient temperature of +20 C°, the gas pressure in the oxygen cylinder should not be higher than 14.7 MPa (150 kgf/cm²) - in accordance with the requirements of GOST 949-73, according to which oxygen cylinders are produced. In the case of oxygen transportation through a pipeline, the oxygen pressure in the pipeline is agreed upon between the supplier and the consumer.
Safety requirements.
Oxygen in cylinders is not a toxic, flammable or explosive gas. Oxygen in cylinders is a strong oxidizing agent that can cause ignition of some materials upon direct contact or increased concentration in the room, which should not exceed 23%. Before inspecting oxygen cylinders or pipelines, purging is performed with ordinary atmospheric air (to reduce the oxygen concentration). Repair of oxygen cylinders or pipelines is also carried out with preliminary purging. When in rooms with high oxygen concentrations (more than 23%), it is strictly forbidden to smoke, turn on heating electrical appliances, or have an open flame, because this contributes to a fire. Premises with high oxygen concentrations must be equipped with ventilation equipment and fire extinguishing equipment. It should be remembered that oxygen cylinders are not intended for storage or transportation of other gases! To maintain product quality, the oxygen cylinder or pipeline must be clean inside and free of foreign contaminants (dust, sand, etc.). When transporting and carrying out loading and unloading operations, the possibility of falling or impact of oxygen cylinders should be excluded. During storage of oxygen cylinders, they must be protected from exposure to direct sunlight, because an increase in temperature increases the gas pressure inside the cylinder. If the gas pressure in the oxygen cylinder is high, the cylinder should be cooled with water.
Rules for oxygen intake.
The sale and delivery of oxygen to the consumer is carried out in batches. The oxygen delivery batch can be any one, with an accompanying quality document.
Rules for returning oxygen cylinders to the supplier.
practices leasing of gas cylinders of various types. When returning a gas cylinder, the consumer must ensure that the residual pressure in the empty oxygen cylinder is not lower than 0.05 MPa (0.5 kgf/cm²).
Dependence of oxygen pressure on temperature during filling, transportation and storage of cylinders
| Temperature, ºС | Working pressure, MPa (kgf/cm²) | Gas pressure at filling temperature, MPa (kgf/cm²) |
| -50 | 9,7 (99) | 12,4 (127) |
| -40 | 10,5 (107) | 13,5 (137) |
| -30 | 11,2 (114) | 14,5 (148) |
| -20 | 11,9 (121) | 15,5 (158) |
| -10 | 12,6 (128) | 16,6 (169) |
| -50 | 9,7 (99) | 12,4 (127) |
| 0 | 13,3 (136) | 17,7 (179) |
| 10 | 14,0 (143) | 18,6 (190) |
| 20 | 14,7 (150) | 19,6 (200) |
| 30 | 15,4 (157) | 20,6 (210) |
Note:
When filling cylinders, as well as storing or transporting filled cylinders at temperatures exceeding those indicated in the table, the gas pressure in the cylinder should not exceed:
Sale and delivery of gas cylinders with oxygen.
supplies enterprises (of various profiles) with technical gases: nitrogen, argon, acetylene, gas mixtures, helium grade “A” and helium grade “B”, technical oxygen, propane, as well as carbon dioxide. In addition to the supply of technical gases, the company specializes in the sale of gas cylinders produced in accordance with GOST 949-73 and GOST 15860-84 (for propane). Among the additional
Source
Application area
Oxygen in cylinders for welding is used in many areas of industry. Almost all places where semi-automatic or gas welding is used require the use of oxygen. With its help, gas-flame processing of metal is carried out, both before and after welding.
If we consider the use of gas for cutting, then it becomes integral, since it is it that gives the high temperature of the jet, which burns through metal products. The minimum acceptable oxygen purity for oxygen use in welding is 99.2%. It is better if this figure is higher when it comes to important work. For home use, there are budget options and 92% oxygen.
New oxygen cylinder
Oxygen is most abundant on Earth. In the earth's crust (about 47% by mass) it exists in a bound form, in the atmosphere (about 23% by mass) - in a free form.
The main ways to obtain oxygen:
Technical gaseous oxygen , according to GOST 5583-78, is produced in two grades: first and second. The oxygen cylinder is painted blue, with the inscription “Oxygen” in black (PB 10-115-96, GOST 949-73). The nominal pressure of gaseous oxygen in the cylinder and auto-recipient at 20°C (GOST 5583-78) is 150 kgf/cm2 (14.7 MPa) or 200 kgf/cm2 (19.6 MPa).
Characteristics of grades of gaseous technical oxygen (GOST 5583-78)
| Parameter | Technical gaseous oxygen | |
| first class | second class | |
| Volume fraction of oxygen O2, %, not less | 99,7 | 99.5 (in some cases – 99.2) |
| Volume fraction of water vapor, %, no more | 0,007 | 0,009 |
| Volume fraction of hydrogen H2, %, no more (only for oxygen obtained by electrolysis of water) | 0,3 | 0,5 |
| Content of carbon dioxide CO2, carbon monoxide CO, gaseous acids and bases, ozone O3 and other oxidizing gases | Not standardized | |
| Alkali content (only for oxygen produced by electrolysis of water) | A piece of filter paper (moistened with a solution of phenolphthalein diluted with water in a ratio of 1:10) in a glass tube with oxygen passed through (0.1–0.2 dm 3 /min for 8–10 minutes) should not turn red or pink. | |
| Smell | Not standardized | |
Permissible oxygen pressure in cylinders depending on temperature (at a nominal pressure of 150 kgf/cm 2 / 20°C)
| Temperature, °C | -50 | -40 | -30 | -20 | -10 | 0 | +10 | +20 | +30 | +40 | +50 |
| Pressure in the cylinder, kgf/cm 2 | 99 | 107 | 124 | 129,5 | 134,5 | 139,5 | 145 | 150 | 155 | 160 | 172 |
To calculate the volume of oxygen gas in a cylinder in m3 under normal conditions, use the formula (GOST 5583-78):
where K1 is the coefficient, Vb is the capacity of the cylinder in dm 3 (l).
Some values of the coefficient K1 for calculating the volume of oxygen gas under normal conditions
| t of gas in the cylinder, °C | K1 value at excess pressure, kgf/cm 2 (MPa) | |||||||||||
| 140 (13,7) | 145 (14,2) | 150 (14,7) | 155 (15,2) | 160 (15,7) | 165 (16,2) | 170 (16,7) | 175 (17,2) | 180 (17,7) | 185 (18,1) | 190 (18,6) | 195 (19,1) | |
| -50 | 0,232 | 0,242 | 0,251 | 0,260 | 0,269 | 0,278 | 0,286 | 0,296 | 0,303 | 0,311 | 0,319 | 0,327 |
| -40 | 0,212 | 0,221 | 0,229 | 0,236 | 0,245 | 0,253 | 0,260 | 0,269 | 0,275 | 0,284 | 0,290 | 0,298 |
| -30 | 0,195 | 0,202 | 0,211 | 0,217 | 0,225 | 0,232 | 0,239 | 0,248 | 0,253 | 0,261 | 0,267 | 0,274 |
| -20 | 0,182 | 0,188 | 0,195 | 0,202 | 0,209 | 0,215 | 0,222 | 0,229 | 0,235 | 0,242 | 0,248 | 0,255 |
| -10 | 0,171 | 0,177 | 0,183 | 0,189 | 0,195 | 0,202 | 0,208 | 0,214 | 0,220 | 0,226 | 0,232 | 0,238 |
| 0 | 0,161 | 0,167 | 0,172 | 0,179 | 0,184 | 0,190 | 0,196 | 0,201 | 0,207 | 0,213 | 0,219 | 0,224 |
| +10 | 0,153 | 0,158 | 0,163 | 0,169 | 0,174 | 0,180 | 0,185 | 0,191 | 0,196 | 0,201 | 0,206 | 0,211 |
| +20 | 0,145 | 0,150 | 0,156 | 0,160 | 0,166 | 0,171 | 0,176 | 0,181 | 0,186 | 0,191 | 0,196 | 0,201 |
| +30 | 0,139 | 0,143 | 0,148 | 0,153 | 0,158 | 0,163 | 0,168 | 0,173 | 0,177 | 0,182 | 0,187 | 0,192 |
| +40 | 0,133 | 0,137 | 0,142 | 0,147 | 0,151 | 0,156 | 0,160 | 0,165 | 0,170 | 0,174 | 0,178 | 0,183 |
| +50 | 0,127 | 0,132 | 0,136 | 0,141 | 0,145 | 0,149 | 0,154 | 0,158 | 0,163 | 0,167 | 0,171 | 0,175 |
Thus, a new cylinder (150 kgf/cm2 at 20°C) with a volume of 40 liters contains 6.24 m3 of oxygen under normal conditions.
Liquid technical oxygen , according to GOST 6331-78, is also produced in first and second grades. It is stored and transported in Dewar flasks, as well as in other cryogenic reservoirs (tanks).
Characteristics of brands of liquid technical oxygen (GOST 6331-78)
When 1 liter of liquid oxygen evaporates, about 860 liters of gaseous oxygen are formed (at normal atmospheric pressure and temperature 20°C). When transporting liquid oxygen, the container weight per 1 kg of oxygen is 10 or more times less than when transporting gaseous oxygen. During storage, transportation and gasification of liquefied gas, losses due to its evaporation are inevitable.
Technical gaseous oxygen: what is it for, types, GOST
Oxygen
Chemical element Boiling liquid oxygen
| Atomic mass | 15.99903 amu |
| Electronic configuration | [He] 2s2 2p4 |
| Electronegativity | 3.44 on the Pauling scale |
| Oxidation states | -2; -1; -0,5; -1/3; 0; +0,5; +1; +2 |
| Density | 0.00142897 g/cm3 |
| Melting temperature | 54.8K |
| Boiling temperature | 90.19 K |
| Crystal lattice structure | monoclinic |
| Thermal conductivity | (300 K) 0.027 W/(m K) |
Chemistry. Oxygen // Science 2.0 [25:38] Oxygen
(lat. Oxygenium
) is a chemical element with atomic number 8. Denoted by the symbol O.
- 1 Description
- 2 History
- 3 Distribution in nature
- 4 Physical properties
- 5 Chemical properties
- 6 Receipt
- 7 Application 7.1 Welding and cutting of metals
- 7.2 In medicine
- 8 In metallurgy
- 8.2 Rocket propellant
- 8.3 Explosives
- 9 Biological role of oxygen
- 10 Toxic oxygen derivatives
- 11 Isotopes
- 12 Facts about oxygen
- 13 Sources
- 14 Literature
[edit] Description
Oxygen is literally “that which produces acid.” The Russian word goes back to M.V. Lomonosov and is a carbon copy of the French word oxygène
, proposed by A. Lavoisier (from ancient Greek ὀξύς - “sour” and γεννάω - “I give birth”).
The atomic number of oxygen is 8; atomic mass - 15.9994. Oxygen forms compounds with all elements except helium, argon and neon. Under normal conditions, oxygen is a gas consisting of diatomic molecules.
At 90.18 K, oxygen condenses into a pale blue liquid, and at 54.36 K it solidifies.
There are other allotropic forms of oxygen, in particular triatomic oxygen (formula O3) called ozone - under normal conditions, a blue gas with a specific odor.
Density of liquid oxygen - 1.144; The melting point is −218 °C, the boiling point is −183 °C.
With some metals, oxygen forms peroxides, superoxides, ozonides, and with flammable gases - explosive mixtures.
The element oxygen ranks third after hydrogen and helium in abundance in the Universe. It is the most abundant chemical element on Earth - 47% of the mass of the earth's crust, 85.7% of the mass of the hydrosphere, 23.15% of the mass of the atmosphere, 79% and 65% of the mass of plants and animals, respectively.
Oxygen occupies 92% of the volume of the earth's crust. About 1,400 minerals containing oxygen are known, the main ones being quartz, feldspars, mica, clay minerals, and carbonates. More than 99.9% of the Earth's oxygen is in a bound state. Oxygen is a factor that regulates the distribution of elements on a planetary scale.
it naturally decreases with depth. The amount of oxygen in igneous rocks varies from 49% in granites to 38-42% in dunites and kimberlites. oxygen in metamorphic rocks corresponds to the depth of their formation: from 44% in eclogites to 48% in crystalline schists. The maximum oxygen is in sedimentary rocks - 49-51%.
An exceptional role in geochemical processes is played by free oxygen - molecular oxygen, the significance of which is determined by its high chemical activity, high migration ability and constant, relatively high content in the biosphere, where it is not only consumed, but also reproduced.
It is believed that free oxygen appeared in the Proterozoic as a result of photosynthesis.
In supergene processes, oxygen is one of the main agents; it oxidizes hydrogen sulfide and lower oxides.
Oxygen determines the behavior of many elements: it increases the migration ability of chalcophiles, oxidizing sulfides to mobile sulfates, and reduces the mobility of iron and manganese, precipitating them in the form of hydroxides and thereby causing their separation.
In ocean waters, the oxygen content changes: in summer the ocean releases oxygen into the atmosphere, in winter it absorbs it. Polar regions are enriched with oxygen. Oxygen compounds, in particular water, are of important geochemical importance.
The main industrial method for producing oxygen is air separation using deep cooling. Oxygen is obtained as a by-product from the electrolysis of water. A method has been developed for producing oxygen by selective diffusion of gases through molecular sieves.
Gaseous oxygen is used in metallurgy to intensify blast furnace and steelmaking processes, in the smelting of non-ferrous metals in shaft furnaces, Bessemer matte, etc. (More than 60% of oxygen consumed); as an oxidizing agent in many chemical industries; in technology - when welding and cutting metals; during underground gasification of coal, etc.
; ozone - for sterilization of drinking water and disinfection of premises. Liquid oxygen is used as an oxidizer for some types of rocket fuel.
oxygen
More than 99.9% of the Earth's oxygen is in a bound state. Oxygen is the main factor regulating the distribution of elements on a planetary scale. it naturally decreases with depth. The amount of oxygen in igneous rocks varies from 49% in acidic volcanics and granites to 38-42% in dunites and kimberlites.
oxygen in metamorphic rocks corresponds to the depth of their formation: from 44% in eclogites to 48% in crystalline schists. The maximum oxygen in sedimentary rocks is 49-51%. When sediments are immersed, they undergo dehydration and partial reduction of iron oxide, accompanied by a decrease in the amount of oxygen in the rock.
When rocks rise from the depths to near-surface conditions, processes of their change begin with the introduction of water and carbon dioxide and the oxygen content increases.
An exceptional role in geochemical processes is played by free oxygen, the importance of which is determined by its high chemical activity, high migration ability and constant, relatively high content in the biosphere, where it is not only consumed, but also reproduced.
[edit] History
Oxygen was discovered in 1773 by the Swedish chemist C. W. Scheele, and independently in 1774 by the English scientist Joseph Priestley. The French chemist Antoine Lavoisier gave the new element its name, and in 1777 he created the oxygen theory of respiration, combustion and oxidation. In its free form it is known as molecular oxygen (O2) and ozone (O3).
Back in the 8th century, the presence of a gas in the air that supports respiration and combustion was established. However, Europeans discovered oxygen almost 1000 years later. The Swedish chemist K.V. Scheele established in 1771 that air consists of oxygen and nitrogen.
An important step that contributed to the discovery of oxygen was the work of the French chemist Pierre Bayen, who published his work on the oxidation of mercury and the subsequent decomposition of its oxide.
In 1774, J. Priestley produced oxygen by decomposing mercuric oxide HgO. But still, the main persons in the history of the discovery of oxygen are not K.V. Scheele and not J. Priestley. They discovered a new gas - oxygen, but despite this, until the end of their lives they remained zealous defenders of the phlogiston theory, which for a long time hampered the development of science.
The works of A. Lavoisier are of particular importance in the history of the discovery of oxygen. In 1775, he established that oxygen is a component of air, created the oxygen theory of combustion (for 200 years it was not only not refuted, but also received many confirmations of its truth), which replaced the theory of phlogiston.
In 1898, the English scientist Thompson, Lord Kelvin, argued that humanity was in danger of suffocation, since huge amounts of carbon dioxide were released into the air not only from breathing, but also from industrial enterprises. This statement was refuted by K. A. Timiryazev.
He proved that green plants that release oxygen during photosynthesis will not allow humanity to die.
[edit] Distribution in nature
Oxygen is the most common element on Earth, its content is 47% by mass (mainly in the form of oxides), in the air (troposphere) oxygen (O2) is 20.93% by volume, or 23% by mass. The composition of water includes 88.8% oxygen, in sea water - 85.7%.
It is part of most rocks, soils, as well as the cells of all plant and animal organisms. Oxygen in general makes up 30-85% of the mass of animal and plant tissues. It is part of proteins, nucleic acids, fats, carbohydrates, etc.
Free oxygen plays an important role in biochemical and physiological processes, in particular in respiration. When there is insufficient supply of oxygen to the body of animals and humans, hypoxia develops.
Green plants and some bacteria are the source of free oxygen on Earth.
In the Universe, oxygen is the third most abundant chemical element after hydrogen and helium.
Being in nature
Accumulation of O2 in the Earth's atmosphere. The green graph is the lower estimate of the oxygen level, the red graph is the upper estimate. 1
. (3.85-2.45 billion years ago) - O2 was not produced
2
. (2.45–1.85 billion years ago) O2 was produced but absorbed by the ocean and seafloor rocks
3
. (1.85-0.85 billion years ago) O2 leaves the ocean, but is consumed during the oxidation of rocks on land and during the formation of the ozone layer
4
. (0.85–0.54 billion years ago) all rocks on land are oxidized, O2 begins to accumulate in the atmosphere
5
. (0.54 billion years ago - present) modern period, O2 content in the atmosphere has stabilized
Hazards and safety precautions
Welding and cutting applications
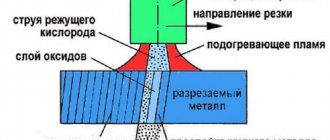
Oxygen is the most important gas for welding and cutting. When a flammable gas is burned in air, a flame is formed with a temperature of no more than 2000°C, and in technically pure oxygen it can exceed 2500–3000°C. It is this flame temperature that is practically suitable for welding many metals.
Flame processing typically uses oxygen with a volume content of 99.2–99.5% or higher. For non-critical types of gas welding, soldering, surface hardening and other methods of heating with a gas flame, oxygen with a purity of 92–98% can be used.
For welding and cutting, oxygen is used in gaseous form, supplied from a cylinder, a gasification plant (SGU-1, SGU-4, SGU-7K, SGU-8K, GKh-0.75, GKhK-3, etc.) or an autonomous station ( KGSN-150, K-0.15, K-0.4, K-0.5, etc.). With significant volumes of consumption, oxygen is safer and more economically feasible to store and transport in liquid rather than gaseous form, despite the inevitable losses during evaporation of liquefied gas.
The conversion of liquid oxygen into gaseous oxygen is carried out in gasification plants - pumped or pumpless. An example of a pumping installation is the stationary installation SGU-1, designed for gasification of non-supercooled oxygen and filling recipients and cylinders under pressure up to 240 kgf/cm 2 (24 MPa).
Along with gas-flame processing processes, oxygen is also used:
New reliable and convenient oxygen cylinder!
The new oxygen cylinder is designed for transportation and storage of gaseous substances.
It is worth noting that the new oxygen cylinder is a classic type of chemical element concentrator. Humanity has been using a special container for several decades. Even leading European countries continue to use this type of equipment.
Currently, the Russian market offers a wide range of oxygen equipment for domestic, industrial and clinical use. Scuba divers always use a new oxygen cylinder when diving under water, craftsmen during welding and work, dentists when treating teeth, etc.
The new oxygen cylinder performs a wide range of serious tasks in each of these areas. That is why a number of important requirements apply to its production. This type of cylinders is made from seamless pipes with compression of the neck and bottom.
The grade of alloy and carbon steels can be different. The stronger the material, the more the container can withstand maximum pressure. The design of the vessel includes a special high-pressure valve, which ensures efficient distribution of oxygen.
The owner of the equipment must be aware of the increased explosion and fire hazard of the new oxygen cylinder. In a standard container, the pressure of the chemical element reaches 200 atmospheres.
In order to prevent an emergency, everyone must fulfill a number of requirements.
First of all, the master must take safety courses on the use of specialized equipment of this type. fill a new oxygen cylinder only in official companies that have a profile and all the necessary licenses.
During operation, it is necessary to properly care for the vessels: constantly monitor the level of oil content, and also degrease the surfaces with alcohol.
In a wide range of products, everyone will find a new oxygen cylinder!
Oxygen storage and transportation
Technical and medical gaseous oxygen is produced in accordance with GOST 5583.
It is stored and transported in steel cylinders GOST 949 under a pressure of 15 MPa. Oxygen cylinders are painted blue with the inscription “OXYGEN” in black letters.
Liquid oxygen is produced in accordance with GOST 6331. O2 is in a liquid state only when received, stored and transported. For gas welding or gas cutting, it must be converted back into a gaseous state.
Where to buy a propane tank
Our company provides you with the opportunity to buy propane at a competitive price.
Our region has a wide network of certified companies selling and refilling cylinders. The most common are tanks with a capacity of 27 and 50 liters, but upon pre-order, you will be supplied with products of 5 or 12 liters in the shortest possible time.
When refueling, of course, it’s faster to exchange your cylinder for an already filled one. However, many people prefer to wait a little to get theirs back. After all, it definitely does not allow gas to pass through and passed the necessary tests on time. The operator himself ensures that the amount of liquid propane does not exceed 85% of the geometric capacity of the vessel. This is necessary so that when heated, the internal pressure does not exceed the permissible threshold. If there are residual contents at the bottom (so-called condensate), the refiller must drain it into a special container.
Rules for the safe operation of propane cylinders
- When using and storing, do not allow the cylinders to overheat (for example, leave them in direct sunlight for a long time);
- It is not recommended to bleed out the propane-butane mixture until the tank is completely empty (under certain conditions it can suck in air, and this is dangerous);
- When transporting, be sure to use plugs and safety caps;
- in case of detection of dents or other defects, the product must be sent for an unscheduled recheck;
- Individuals are allowed to transport no more than five cylinders in one vehicle (they must be separated by spacers from each other).
- It is necessary to constantly monitor the condition of the cylinders, because it is not for nothing that they are considered fire and explosive objects.
What is the gas pressure in a propane tank?
According to GOST 15860-84, the working pressure in the tank should not exceed 1.6 MPa. In this case, the proportion of propane in the hydrocarbon mixture must be no less than 60%. This is very important for the safe operation of gas cylinder installations. Of course, products are designed for significantly higher pressures - more than 5.0 MPa. Production and periodic tests are carried out under a pressure of 3.0 MPa.
Refueling standards
At gas cylinder refilling stations, employees are familiar with the regulations. Since a cylinder that is filled too much can explode or its valve can be torn off. So, if you refuel from a reliable supplier, you have nothing to worry about.