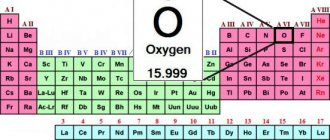
- Designation - O (Oxygen);
- Latin name - Oxigenium;
- Period - II;
- Group - 16 (VIa);
- Atomic mass - 15.9994;
- Atomic number - 8;
- Atomic radius = 60 pm;
- Covalent radius = 73 pm;
- Electron distribution - 1s22s22p4;
- melting temperature = -218.4°C;
- boiling point = -182.96°C;
- Electronegativity (according to Pauling/according to Alpred and Rochow) = 3.44/3.50;
- Oxidation state: +2; +1; 1/2; 0; -1/3; -1/2; -1; -2;
- Density (no.) = 1.42897 g/cm3;
- Molar volume = 14.0 cm3/mol.
Oxygen (“giving birth to acids”) was discovered in 1774 by J. Priestley.
This is the most common chemical element on Earth - the mass fraction of oxygen in the earth's crust is 47.2%. In atmospheric air, the proportion of oxygen is 21%, which is associated with the activity of green plants. Oxygen is part of many, both inorganic and organic compounds. Oxygen is necessary for the life of all highly organized living organisms: humans, animals, birds, fish. Oxygen makes up from 50 to 85% of the mass of animal and plant tissues.
Three stable isotopes of oxygen are known: 16O, 17O, 18O.
In the free state, oxygen exists in two allotropic modifications: O2 - oxygen; O3 - ozone.
Oxygen in D.I. Mendeleev’s Periodic Table of Chemical Elements is numbered “8” and belongs to the 16(VIa) group (See Atoms of the 16(VIa) group).
Rice. The structure of the oxygen atom.
The oxygen atom contains 8 electrons: 2 electrons are in the inner s-orbital and 6 more in the outer energy level - 2 (paired) in the s-sublevel and 4 (two paired and two unpaired) in the p-sublevel (see Electronic structure of atoms) .
Due to two unpaired p-electrons of the outer level, oxygen forms two covalent bonds, accepting two electrons and exhibiting the oxidation state -2 (H2O, CaO, H2SO4).
In compounds with an O-O oxygen bond, the oxygen atom exhibits the oxidation state -1 (H2O2).
With the more electronegative fluorine, oxygen gives up its valence electrons, exhibiting the +2 oxidation state (OF2).
General information
Oxygen is a gaseous chemical element. It is designated by the symbol O, has an atomic number of 8 and an atomic weight of 15.9994, and its molar mass is 32 g/mol. Oxygen formula O2. The diagram of the electronic configuration of the oxygen atom is 1s 2 2s 2 2p 4. The structure of the oxygen atom has two shells, like all elements located in the second period.
It is of great interest because it is an essential element in the respiratory processes of most living cells and combustion processes. It is the most common element in the earth's crust. Almost one-fifth (by volume) of air is O2. Oxidation state -2.
Under normal conditions, O2 is a colorless, odorless and tasteless gas. It condenses into a light blue liquid. It is a reactive element that forms oxides with all other elements except helium, neon, argon and krypton. It is moderately soluble in water (30 cm3 per 1 liter of dissolving water) at a temperature of 20 degrees Celsius.
Classes and nomenclature of inorganic substances
Nomenclature is a way of naming substances.
Chemical formula is a representation of the composition of a substance using symbols of chemical elements, numerical indices and other symbols. The chemical name is determined by the composition of the substance and is depicted using a word or group of words. The names are constructed according to nomenclatural rules, using Russian names of elements, except in cases where Latin roots are traditionally used (Table 3):
| Ag - argent | C - carb, carbon | H - hydr, hydrogen | N - nitr | Pb - plumb, | Si - silic, silic, silic |
| As - ars, arsen | Cu - cupr | Hg - mercury | Ni - nikkol | S - sulf | Sn-stann |
| Au - aur | Fe - ferr | Mn - mangan | O - ox, oxygen | Sb - stib | |
| For example, sodium oxide Na2O, calcium carbonate CaCO3, potassium permanganate | |||||
- The names of simple substances most often coincide with the Russian names of the corresponding chemical elements. If necessary, a numerical Greek prefix is added to them: mono - 1, di (Latin) - 2, three - 3, tetra - 4, penta - 5, hexa - 6, hepta - 7, octa - 8, nona (Latin) - 9 , deca - 10. For example, (mono) calcium Ca, (mono) copper Cu, dioxygen O2, trioxygen O3, tetraphosphorus P4. Exception: allotropic modifications: carbon C - graphite, soot, diamond; oxygen - ozone O3.
- The names of complex substances are composed according to the chemical formula from right to left. Each class of substance has its own rules for compiling formulas and names:
- oxide formula : EnOm, where n and m are numerical indices determined by the oxidation states of the elements. For example,
Li+1 and O-2→ Li2O; Al+3 and O-2→ Al2O3; N+5 and O-2→ N2O5.
Name of the oxide: the word “oxide” in the nominative case + the name of the element E in the genitive case: lithium oxide Li2O, aluminum oxide Al2O3.
If an element forms several oxides, then add the oxidation number in Roman numerals at the end, enclosing them in brackets:
- P2O5 - (di)phosphorus pentoxide or phosphorus (V) oxide, read: “phosphorus oxide five”;
- Fe2O3 is iron (di)trioxide or iron (III) oxide, read: “iron oxide three.”
Oxides that correspond to acids are also called anhydrides: sulfuric anhydride SO3, nitric anhydride N2O5, etc.
- base formula : Me+n(OH-)n, where the subscript n is the number of hydroxide anions OH-.
K+1 and OH- → KOH, Mg+2 and OH- → Mg(OH)2.
Name: the word “hydroxide” in the nominative case + the name of the element in the genitive case: potassium hydroxide, magnesium hydroxide.
If an element forms several hydroxides, then add the oxidation number in Roman numerals at the end, enclosing them in brackets:
Fe(OH)2 is iron (II) hydroxide, Cr(OH)3 is chromium (III) hydroxide.
- acid formula HnK, where K is the acid residue.
Names of oxygen-free acids: the root of the Russian name of the element that forms the acid + the suffix “o” + “-hydrogen acid”, for example: HBr - hydrobromic acid, HCl - hydrochloric acid, H2S - hydrosulfide acid.
Names of oxygen-containing acids: Russian name of the forming element + “acid”, taking into account the rules:
- If the element is in the highest oxidation state, then the ending will be “-th” or “-ova”: H2SO4 is sulfuric acid, H3AsO4 is arsenic acid. The ending changes with decreasing oxidation degree in the sequence: “-ovate” (HClO3—hypochlorous acid), “-clear” (HClO2—chlorous acid), “-ovate” (HClO—hypochlorous acid).
- If more than one acid corresponds to an oxide, then the prefix “meta” is added to the name of the acid with the minimum number of oxygen atoms, and “ortho” is added to the name of the acid with the maximum number of oxygen atoms, for example, HPO3 - metaphosphoric acid, H3PO4 - orthophosphoric acid.
The names of the most common acids and their residues are given in Table 4:
| Formula and name of acid | Name of the acid residue that forms the salt |
| HAlO2 meta-aluminum | metaaluminate |
| H3AlO3 orthoaluminum | orthoaluminate |
| HAsO3 metaarsenic | metaarsenate |
| H3AsO4 orthoarsenic | orthoarsenate |
| H3BO3 orthoboric | orthoborate |
| HBr hydrogen bromide | bromide |
| HBrO bromide | hypobromite |
| HBrO3 brominated | bromate |
| HCN hydrogen cyanide | cyanide |
| H2CO3 coal | carbonate |
| HCl hydrochloric | chloride |
| HClO hypochlorous | hypochlorite |
| HClO2 chloride | chlorite |
| HClO3 chloric | chlorate |
| HClO4 chlorine | perchlorate |
| HF hydrogen fluoride | fluoride |
| HJ hydrogen iodide | iodide |
| HMnO4 manganese | permanganate |
| HNO2 nitrogenous | nitrite |
| HNO3 nitrogen | nitrate |
| HPO3 metaphosphoric | metaphosphate |
| H3PO4 orthophosphoric | orthophosphate |
| H2S hydrogen sulfide | sulfide |
| H2SO3 sulfurous | sulfite |
| H2SO4 sulfuric | sulfate |
| H2SiO3 metasilicon | metasilicate |
| H3SiO4 orthosilicon | orthosilicate |
- salt formula : MemKn
The name is formed depending on the type of salt.
- Medium salts - the name of the acidic residue in the nominative case + the name of the cation in the genitive case, if necessary, the oxidation state is added: sodium chloride NaCl, copper (II) sulfate CuSO4, etc.
- Acidic (only for polybasic acids) - the prefix “hydro”, if necessary, add a numerical value (di-, tri-, tetra-, etc.) + name of the acid residue + name of the cation: sodium bicarbonate NaHCO3, barium dihydrogen orthophosphate Ba(H2PO4 )2.
- The main ones are the prefix “hydroxo” with a numerical value, if necessary + the name of the acid residue + the name of the cation: magnesium hydroxychloride MgOHCl, iron (III) dihydroxychloride Fe(OH)2Cl.
- Double - anion in the nominative case + hyphenated cations in the genitive case: ammonium-magnesium orthophosphate NH4MgPO4; aluminum-lithium metasilicate LiAl(SiO3)2.
- Mixed - the name of anions with a hyphen in the nominative case + the name of the cation in the genitive case: calcium chloride-hypochlorite Ca(ClO)Cl; sodium nitrate iodate Na2IO3(NO3).
- Complex - the name of the cation in the nominative case + the name of the anion in the genitive case: diammine silver (I) chloride [Ag(NH3)2]Cl; sodium tetrahydroxoaluminate Na[Al(OH)4].
- nomenclature of binary compounds.
History of discovery
What is air? Ancient peoples thought deeply about this issue. And this is not surprising when you realize how important air is for many processes. Objects cannot burn without air. Man cannot survive without air. In fact, ancient peoples thought that air was supposed to be an "element." But they used the word “element” in a slightly different meaning than modern scientists. For ancient people, the element was something very important and basic. Air fits this description, along with fire, water and earth. Subsequently, many scientists influenced the discovery of such an element as oxygen:
- The first person in Western Europe to describe the “parts” of air was the Italian artist and scientist Leonardo da Vinci (1452−1519). Leonardo noted that air is not completely consumed when something is burned in it. Therefore, he said that air must consist of two parts: one part that is spent on combustion, and one part that does not participate in the process. For many years, Leonardo's ideas were not very popular among scientists. The problem was that early chemists did not have good equipment like modern laboratories. They found it difficult to collect air samples and then study it.
- In the early 1700s, chemists began to learn more about air, but in a somewhat roundabout way. For example, in 1771 and 1772, Karl Wilhelm Scheele studied the effect of heat on a number of compounds. In one experiment, he used silver carbonate (Ag 2 CO 3), mercuric carbonate (HgCO 3) and magnesium nitrate (Mg(NO 3) 2). When he heated these compounds, he found that gas was released. He then studied the properties of this gas and found that the flame burned brightly in it. He also noticed that animals could stay in it without pain. Without knowing this, Scheele discovered oxygen.
- About two years later, Joseph Priestley conducted similar experiments by heating mercuric oxide (HgO) in a flame. The compound disintegrated, resulting in the formation of liquid metallic mercury and gas. When Priestley tested the new gas, he found the same properties and characteristics as Scheele. Priestley even tried to inhale the gas that he managed to obtain.
- Antoine-Laurent Lavoisier (1743−94) is often called the father of modern chemistry. He received this title for a number of reasons. The most important of these is the explanation he discovered for the process of combustion (burning). Before Lavoisier's research, chemists believed that a burning object released a substance into the air. They called this substance phlogiston. For example, when a tree burned, chemists said that phlogiston moved from the tree into the air. Lavoisier showed that this idea was incorrect. When something burns, it is actually reacting with oxygen in the air. Combustion, Lavoisier said, is simply oxidation (the process by which a substance combines with O2). This discovery gave chemists a completely new way of looking at chemical changes. The phlogiston theory gradually began to die out. Many ideas began to develop that are used today in modern chemistry.
Some people think that Scheele should be given credit for the discovery of oxygen. He completed his experiments before Priestley. But his publisher was very slow in printing the scientist’s reports. They actually came out after Priestley's reports. Thus, most historians agree that Scheele and Priestley should share the right to discover oxygen.
Neither Scheele nor Priestley fully understood the importance of their discovery. This step was taken by the French chemist Antoine-Laurent Lavoisier (1743−94). Lavoisier was the first person to declare that the new gas was an element. He was also the first person to explain how oxygen is involved in combustion. In addition, he suggested a name for the gas.
The word oxygenium (“oxygen”) comes from Greek words meaning “acid-producing.” Lavoisier chose this name because he thought that all acids contain O2. Therefore, the new element was responsible for the “formation of acids.” However, in this respect Lavoisier was mistaken. Not all acids contain oxygen.
Element in the environment
The Earth's crust is composed primarily of silicon-oxygen minerals, and many other elements are present as their oxides. Oxygen gas makes up one fifth of the atmosphere. O2 in the Earth's atmosphere is produced by photosynthesis by plants , and accumulated over long periods of time as they used the abundant supplies of carbon dioxide in the early atmosphere and released oxygen.
The element is highly soluble in water, which makes life possible in rivers, lakes and oceans. The water in these bodies of water must be regularly supplied with oxygen because when its supply of O2 is depleted, it can no longer support fish and other aquatic organisms.
Almost all chemicals, except noble gases, combine with oxygen to form compounds. Water, H2O, and silica, SiO2, the main component of sand, are among the most common double oxygen compounds. Among compounds that contain more than two elements, the most common are silicates, which form most rocks and soils. Other compounds that occur in abundance in nature are calcium carbonate (limestone and marble), calcium sulfate (gypsum), aluminum oxide (bauxite) and various iron oxides, which are used as a metal source.
The element is found in all types of minerals. Some common examples include oxides, carbonates, nitrates, sulfates and phosphates. Oxides are chemical compounds that contain oxygen and another element. Carbonates are compounds that contain oxygen, carbon and one other element. An example is sodium carbonate or soda, soda ash or salt soda (Na2CO3), which is often found in detergents and cleaning products.
Nitrates, sulfates and phosphates also contain oxygen. Other elements in these compounds are nitrogen, sulfur or phosphorus plus one other element. Examples of these compounds are potassium nitrate or saltpeter (KNO3), magnesium sulfate or Epsom salts (MgSO4) and calcium phosphate (Ca3(PO4)2).
Classification of simple substances
1. Simple substances are conventionally divided into two groups: metals and non-metals.
Nonmetals
in the Periodic Table - these are all elements of group VIII A (noble gases) and group VII A (halogens), elements of group VI A (except polonium), elements of group V A: nitrogen, phosphorus, arsenic;
carbon, silicon (IV A-group); boron (III A-group), as well as hydrogen. The remaining elements are classified as metals .
The differences in the properties of metals and non-metals are shown in Table 1:
| metals | nonmetals | ||
| Type of chemical bond | metal | covalent nonpolar | |
| Crystal cell | metal | atomic or molecular | |
| Physical properties | State of aggregation | solid, except liquid mercury Hg |
|
| Shine | metallic shine | do not have shine (exception: iodine J2 and graphite) | |
| Ability to conduct heat and electricity | good guides | conduct heat poorly, do not conduct current - dielectrics (exception: graphite, silicon Si and black phosphorus) | |
| Strength, malleability, ductility | characteristic of all metals (exception: chromium Cr, manganese Mn, antimony Sb) | brittle when solid | |
| Color | silver-white, silver-gray (exception: red copper Cu, yellow gold Au and some others) | various: almost black iodine J2, yellow sulfur S, black, white and red phosphorus P, colorless oxygen O2, nitrogen N2 | |
| Allotropic ability | weak; some metals: iron Fe, tin Sn, lanthanides and actinides. | good; carbon C has many modifications (graphite, fullerene, diamond, carbyne, etc.); phosphorus P (white, black, red); sulfur S (crystalline, plastic) | |
| Allotropy is the ability of some elements to exist in the form of two or more simple substances (allotropic modifications), differing in structure and properties. | |||
Amphoteric elements are found in the A-groups of the Periodic Table: beryllium Be, aluminum Al, gallium Ga, germanium Ge, tin Sn, lead Pb, antimony Sb, bismuth Bi, polonium Po, etc., as well as most B-group elements: chromium Cr , manganese Mn, iron Fe, zinc Zn, cadmium Cd, gold Au, etc., exhibit both metallic (basic for compounds) and non-metallic (acidic for compounds) properties.
Noble (inert) gases (VIII A-group of the Periodic Table): helium He, neon Ne, argon Ar, krypton Kr, xenon Xe and radioactive radon Rn:
- found in the air, in small quantities - in water, rocks, natural gases;
- have no color, taste or smell;
- extremely chemically inert;
- used in light sources to create lighting of various colors (Ne - fiery red, Xe - bluish-gray, dim, Ar - violet-blue, etc.).
2. Complex compounds and their differences from simple substances.
Complex substances are organic , based on carbon, and inorganic (carbon-free and some carbon-containing compounds: carbides, carbonates, carbon oxides and others). Inorganic compounds are most often divided into oxides, bases, acids and salts.
The main differences between complex inorganic substances:
- The properties of the elements included in the connection are not saved. For example, the metal calcium Ca and the nonmetal chlorine Cl2. Each of these simple substances has its own characteristics. And the salt CaCl2 has new properties, different from the characteristics of simple substances, similar to the properties of the class of salts.
- Through chemical reactions, a complex substance can be formed or broken down into its component parts.
- The quantitative composition of a complex compound is always the same, regardless of location and method of preparation (for substances of molecular composition).
Isotopes of oxygen
There are three naturally occurring isotopes of O2 : oxygen-16, oxygen-17 and oxygen-18. Isotopes are two or more forms of an element. They differ from each other by their mass number. The number written to the right of the element's name is the mass number. It represents the number of protons plus neutrons in the nucleus of an element's atom. The number of protons defines an element, but the number of neutrons in an atom of any one element can vary. Each variation is an isotope.
There are also five known radioactive isotopes of the element. A radioactive isotope is one that breaks apart and emits some form of radiation. Radioactive isotopes are formed when very small particles burn atoms. These particles stick to atoms and make them radioactive.
What is m in chemistry?
, where m is the mass of the substance, M is the molar mass of the substance. Molar mass is the mass per mole of a given substance. The molar mass of a substance can be obtained by multiplying the molecular mass of this substance by the number of molecules in 1 mole - by Avogadro's number.
Interesting materials:
How does Avito delivery collection points work? How does a water vending machine work? How does an automatic needle threader work? How does the kettle automatically turn off work? How does the iron's automatic shut-off work? How does a self-service car wash work in winter? How does authorization through cookies work? How does a load balancer work? How does hair conditioner work? How does a gasoline generator work?
Health effects
Oxygen is essential to all life forms as it is a constituent of DNA and almost all other biologically important compounds. In the lungs, this element is absorbed by the iron atom in the center of hemoglobin in the blood and is thus transported to where it is needed.
Every person needs this element to breathe, but, as with many things, too much of it is harmful. If a person is exposed to large amounts of O2 for a long time, lung damage can occur. Inhaling 50-100% oxygen at normal pressure over an extended period causes lung damage. People who work with frequent or potentially high exposure to pure elements should undergo pulmonary function tests before starting work and upon completion.
Test on the topic
- /5
Question 1 of 5How many electrons does an oxygen atom have?
Start test
Hall of Fame
To get here, take the test.
- Alexander Kotkov
5/5
- Mila Kondratieva
5/5
- Semyon Goldfarb
5/5
- Alexander Kotkov
5/5
Chemical properties
At standard temperature and pressure, two atoms of an element bond to form oxygen dioxide, a colorless, odorless, and tasteless diatomic gas with the formula O2. This element is a member of the chalcogen group of the periodic table and is a highly reactive non-metallic element. It easily forms compounds (particularly oxides) with almost all other elements.
Oxygen is a strong oxidizing agent and has the second highest electronegativity of all reactive elements, second only to fluorine. By mass, oxygen is the third most abundant element in the universe after hydrogen and helium and the most abundant element by mass in the Earth's crust, accounting for almost half the mass of the Earth's crust. Free oxygen is chemically reactive to appear on Earth without the photosynthetic action of living organisms, which use the energy of sunlight to produce elemental oxygen from water.
Elemental O2 began to accumulate in the atmosphere only after the evolutionary emergence of photosynthetic organisms, approximately 2.5 billion years ago. Diatomic oxygen gas currently makes up 20.8% of air volume.
The most important chemical properties of oxygen are:
- Maintaining combustion. It helps other objects burn. Burning charcoal is an example. Charcoal is almost pure carbon.
- Connects to elements at room temperature. Rust is an example. Rust is a process by which metal combines with O2.
- Reacts with many compounds. Decay is the process by which once living matter combines with oxygen. The breakdown products are mainly carbon dioxide (CO2) and water (H2O):
O2 itself does not burn. A lit match in a cylinder with a pure element burns much brighter, but the oxygen does not ignite.
Note:
100* The data in the table is based on oxygen (O2).
201* The range of atomic mass values is indicated due to the varying abundance of isotopes of a given element in nature.
206* The covalent radius of oxygen according to [1] is 66±2 pm.
401* The density of oxygen according to [1] is 0.001429 g/cm3 (at 0 °C and other standard conditions, the state of the substance is gas).
402* The melting point of oxygen according to [3] is -218.35 °C (54.8 K, -361.03 °F).
403* The boiling point of oxygen according to [3] is -182.96 °C (90.19 K, -297.33 °F).
407* The specific heat of fusion (enthalpy of fusion ΔHmelt) of oxygen according to [4] is 0.446 kJ/mol.
408* The specific heat of evaporation (enthalpy of boiling ΔHboil) of oxygen according to [3] and [4] is 3.4099 kJ/mol and 6.828 kJ/mol, respectively.
410* The molar heat capacity of oxygen according to [3] is 29.4 J/(K mol).
415* The critical temperature of oxygen according to [4] is -118.37 °C (154.78 K, -181.07 °F).
416* The critical oxygen pressure according to [4] is 5.08 MPa, respectively.
Physical properties
Oxygen is more soluble in water than nitrogen. Water contains approximately one O2 molecule for every two N2 molecules, compared to an atmospheric ratio of approximately one to four. The density of oxygen in the gaseous state is 1.42897 kg/m3. Other physical properties of the element are:
- The gas in its normal state is colorless, odorless and tasteless. Liquid oxygen is slightly paramagnetic. It is reactive and forms oxides with all elements except helium, neon, krypton and argon. Moderately soluble in water.
- The element condenses at a temperature of -182.95C and freezes at -218.79C. In both liquid and solid states it is a transparent substance of light blue color.
- The existence of an element in two allotropic forms. Allotropes are modifications of an element with different physical and chemical properties. Allotropes of O2 are diatomic oxygen and ozone, or triatomic. Ozone occurs in fairly large quantities under special conditions. For example, unusually large amounts of ozone are found in the Earth's upper atmosphere. This ozone layer is important for life on the planet. It protects against harmful radiation that comes from the Sun. Ozone at ground level is not useful for life and can cause health problems in plants, people and animals.
- Atomic oxygen is very highly reactive. It is capable of oxidizing atoms of elements that are unusual for this organism, and is one of the strongest antioxidants that eliminates oxygen starvation of tissues and destroys almost any pathogenic microflora (fungi, viruses, bacteria and others) and excess free radicals.
Prevalence in nature.
K. is the most common chemical. element on Earth: the content of chemically bound carbon in the hydrosphere is 85.82% (mainly in the form of water), in the earth’s crust - 49% by weight. More than 1,400 minerals are known that contain K. Among them, the predominant minerals are those formed by salts of oxygen-containing acids (the most important classes are natural carbonates, natural silicates, natural sulfates, natural phosphates), and rocks based on them (for example, limestone, marble ), as well as various natural oxides, natural hydroxides and rocks (eg basalt). Molecular carbon makes up 20.95% by volume (23.10% by mass) of the earth's atmosphere. K. atmosphere has a biological origin and is formed in green plants containing chlorophyll from water and carbon dioxide during photosynthesis. The amount of potassium released by plants compensates for the amount of potassium consumed in the processes of decay, combustion, and respiration. K. - a biogenic element - is part of the most important classes of natural organics. compounds (proteins, fats, nucleic acids, carbohydrates, etc.) and in the composition of inorganic. skeletal connections.
Application of oxygen
Molecular dioxide O2 is essential for cellular respiration in all aerobic organisms. Its reactive species, such as superoxide ion (O2-) and hydrogen peroxide (H2O2), are dangerous byproducts of oxygen use in organisms. However, parts of the immune system of higher organisms use reactive peroxide, superoxide, and singlet oxygen to destroy invading microbes. Reactive species also play an important role in the hypersensitive response of plants to pathogenic microorganisms.
At rest, an adult inhales between 1.8 and 2.4 g of oxygen per minute. This amounts to more than 6 billion tons of the element inhaled by humanity per year. Areas of use include the following:
- People who have breathing problems use oxygen masks and tanks to get the oxygen they need.
- It is used in rocket fuel, combined with hydrogen in the engine. When hydrogen and oxygen combine, they release very large amounts of energy. The energy is used to launch a rocket into space.
- Metal production accounts for the largest percentage of O2 use. For example, the element is used to burn carbon and other impurities found in iron to produce steel. A small amount of these impurities can be beneficial to the steel, but too much makes it brittle and unusable. Carbon and other impurities are burned off during steel production by blowing O2 through molten iron.
- Used in the production of metals such as copper, lead and zinc. These metals occur in the earth in the form of sulfides, such as copper sulfide (CuS), lead sulfide (PbS) and zinc sulfide (ZnS). The first step in extracting these metals is to convert them into oxides. The oxides are then heated with carbon to produce pure metals.
- It is used in the chemical industry as a starting material for the production of some very important compounds. Sometimes the stages of transition from oxygen to the final compound are lengthy. For example, ethylene gas (C2H4) can be treated with oxygen to form ethylene oxide (CH2CH2O). About 60% of the resulting ethylene oxide is converted to ethylene glycol (CH2CH2 (OH)2). Ethylene glycol is used as an antifreeze and serves as a starting point in the production of polyester fibers, films, plastic containers, bags and packaging materials
- Used in oxyacetylene welding, as an oxidizer for rocket fuel, and in the production of methanol and ethylene oxide.
- Plants and animals use it for respiration.
- Pure oxygen is often used to help patients with respiratory problems breathe easier.
Oxygen and its compounds play a key role in many important processes in life and industry.
Receipt
In prom. On a large scale, water is produced by liquefying and fractional distillation of air (see Art. Air separation), as well as by electrolysis of water. In laboratory conditions, K. is obtained by decomposition when heating hydrogen peroxide (2H2O2 = 2H2O+ O2), metal oxides (for example, mercury oxide: 2HgO = 2Hg + O2), salts of oxygen-containing oxidizing acids (for example, potassium chlorate: 2KClO3 = 2KCl +3O2, potassium permanganate: 2KMnO4=K2MnO4+MnO2+O2), by electrolysis of an aqueous solution of NaOH. Gaseous K. is stored and transported in steel cylinders, painted blue, at a pressure of 15 and 42 MPa, liquid K. - in metallic ones. Dewar vessels or special tank tanks.