How much gas can you fill in a 50 liter cylinder?
A 50-liter propane cylinder holds no more than 42.5 liters of liquefied gas. The fact is that according to safety standards, no more than 85 percent of the total internal volume can be pumped into the container (50x85% = 42.5).
Interesting materials:
Is it possible to sprinkle ash on fruit trees? Is it possible to hang two birdhouses on one tree? Is it possible to nail a birdhouse to a tree? Is it possible to graft trees in August? Is it possible to cut down trees on your property? Is it possible to cut down trees on agricultural land? Is it possible to cut down trees in the forest? Can I cut down a tree in my yard myself? Is it possible to plant fruit trees in May? Is it possible to plant fruit trees in November?
Calculation of propane-butane in cylinders
The parameters and dimensions of oxygen cylinders for propane, butane and their mixtures can be viewed according to GOST 15860-84. Currently, four types of these products are used, with volumes of 5, 12, 27 and 50 liters.
Under normal atmospheric conditions and a temperature of 15°C, the density of propane in the liquid state is 510 kg/m3, and butane 580 kg/m3. Propane in the gas state at atmospheric pressure and temperature 15°C is 1.9 kg/m3, and butane is 2.55 kg/m3. Under normal atmospheric conditions and a temperature of 15°C, 0.392 m3 of gas is formed from 1 kg of liquid butane, and 0.526 m3 from 1 kg of propane.
Dimensions of methane cylinders of the third type
Model: CNG-3 STAKO
| Volume, l | Outer diameter, mm | Length, mm | Weight, kg |
| 47 | 326 | 860 | 33,6 |
| 50 | 326 | 900 | 35,2 |
| 67 | 326 | 1140 | 44,6 |
| 80 | 326 | 1360 | 53,2 |
| 100 | 326 | 1660 | 65 |
| 123 | 326 | 2000 | 78,4 |
| 67 | 398 | 840 | 45,6 |
| 80 | 398 | 965 | 52,3 |
| 85 | 398 | 1015 | 55 |
| 96 | 398 | 1125 | 61,1 |
| 100 | 398 | 1165 | 63,5 |
| 132 | 398 | 1485 | 80,5 |
| 160 | 398 | 1765 | 99,5 |
| 185 | 398 | 2005 | 108,5 |
Application of the Mendeleev-Clapeyron equation in calculating the volume and quantity of gaseous substances
Problem 36. Calculate what volume (in liters) is occupied by: a) 1.2 kg of water vapor at 100 °C and 1.013 105 Pa; b) 1.2 kg of methane at 25 °C and 1.013 105 Pa. Solution: M[H2O(vapor)] = 18 kg/mol . 10-3; M(CH4) = 16 kg/mol . 10-3; T1 = 100 °C = (100 + 273) = 373 K; T2 = (25 + 273 = 298 K); P1 = P2 = 1.013 105 Pa.
To solve the problem, let's apply the Mendeleev-Clapeyron equation:
PV = nRT = mRT/M, where
n is the number of moles of gas; P – gas pressure (for example, in atm or Pa; V – gas volume (in liters); T – gas temperature (in kelvins); R – gas constant [0.0821 l atm/mol K)] or [8.314 J/(mol K )]; M is the molar mass of the substance (in g/mol or kg/mol; m is the mass of the substance (for example, in g or kg).
Let's calculate the volumes of gases:
a) volume of 1.2 kg of water vapor:
PV = mRT/M, V(par) = mRT1/MP = [1,2 . 8.314 J/(mol K ) . 373 K]/[(18 kg/mol . 10-3) . 1.013·105 Pa] = = 3721.3464/1823.4 = 2.04 m3 = 2040 l.
b) volume of 1.2 kg of methane:
V(CH4) = mRT1/MP = [1,2 . 8.314 J/(mol K ) . 298 K]/[(16 kg/mol 10-3 ) * 1.013 105 Pa] = = 2973.0864/1620.8 = 1.834 m3 = 1834 l.
Answer: V(steam) = 2040 l; V(CH4) = 1834 l.
Problem 37. A certain amount of helium gas at 78 °C and a pressure of 15.6 atm occupies a volume of 26.5 liters. What is the volume of this gas under normal conditions? How many moles of helium is this? Solution: To solve the problem, let’s apply the Mendeleev-Clapeyron equation:
PV = nRT, where
n is the number of moles of gas; P – gas pressure (for example, in atm or Pa; V – gas volume (in liters); T – gas temperature (in kelvins); R – gas constant [0.0821 l atm/mol K)] or [8.314 J/(mol K )].
The Clapeyron-Mendeleev equation is equally valid for both the initial state of the gas and the final one:
P1V1 = nRT1; P2V2 = nRT2.
If we divide the upper equation by the lower equation term by term, then with a constant number of moles n we get:
(P1V1 = nRT1)/(P2V2) = nRT2) = (P1V1)/(P2V2) = T1/T2;
Then
V2(He) = P1V1Т2/P2T1 = (15.6·26.5·273)/(351.1 ) = 112858.2/351 = 321.5 l.
Let's find the number of moles of helium:
n(He) = V/Vm = 321.5/22.4 = 14.35 mol.
Answer: V2(He) = 321.5 l; n(He) = 14.35 mol.
Problem 38. A steel cylinder with a volume of 40 liters contains hydrogen under a pressure of 60 atm and a temperature of 25 °C. How many moles of hydrogen are in the cylinder? How many grams? What volume will hydrogen occupy from the cylinder at zero conditions? Solution: M(H2) - 2 g/mol; V1 = 40 l; P1 - 60 atm; T1 = T0 = 25 °C = 298 K; P0 = 1 atm. n(H2) = ? m(H2) = ? V0(H2) = ?
To solve the problem, let's apply the Mendeleev-Clapeyron equation:
PV = nRT, where
n is the number of moles of gas; P – gas pressure (for example, in atm or Pa; V – gas volume (in liters); T – gas temperature (in kelvin); R – gas constant [0.0821 l atm/mol K)] or [8.314 J/(mol / K)].
Let's calculate how many moles of hydrogen are in the cylinder, we get:
PV = nRT, n = PV/RT;
n(H2) = P1V1/RT1 = (60.40 ) /(0.0821.298 ) = 2400/24.4658 = 98.1 mol.
Find the mass of hydrogen in the balloon:
m(H2) = n(H2) . M(H2) = 98.1 . 2 = 196.2 g.
Let's calculate the volume of hydrogen from the cylinder (n.s.), we get:
PV = nRT, V = nRT/P;
V0(H2) = n(H2)RT0/P0 = (98.1 . 0.0821 . 298)/1 = 2400 l.
Answer: n(H2) = 98.1 mol; m(H2) = 196.2 g; V0(H2) = 2400 l.
Pressure gauge
On large tanks, a pressure detection apparatus must be installed. You can determine the amount of fuel in the tank by multiplying the volume of the cylinder by the pressure gauge values. In this way, the value is determined only approximately, since the internal pressure is significantly influenced by the properties of the contents and the external air temperature.
Source ru.wikipedia.org
Application: gas treatment
Long-term and intermediate storage of cylinders is allowed on ramps equipped with roofs and protective partitions that prevent the ingress of precipitation, in cold and heated rooms with natural ventilation.
Liquid carbon dioxide supplied for welding work is purchased in the highest and first grades . Refilling cylinders with carbon dioxide for food producers is expensive, but desirable: The gas humidity is zero.
The use of second grade gas is allowed if drying is possible: an unregulated amount of liquid vapor is added to 1% of the aqueous sediment. A gas dryer extracts water vapor from a gas flow.
This is a sealed container filled with hygroscopic materials. Low pressure dryers are installed after the reducer, high pressure dryers take gas from a cylinder in front of the reducer. Desiccants are aluminum gel, silica gel, and copper sulfate .
Adiabatic cooling of a gas provokes a sharp volumetric expansion. Gas consumption in the range of 15–20 l/min leads to freezing of moisture vapor, which can lead to clogging of the gearbox. A high-volume gas intake requires the installation of a 24/36 V coil-type gas heater. The thermoelement neutralizes the freezing of water vapor and is designed to pass large volumes.
Active gas protection of welds during semi-automatic arc welding with a consumable wire electrode is carried out with carbon dioxide in pure form or mixed with argon.
The use of cylinders implies a limited daily consumption by welding stations . A 40-liter cylinder with an internal pressure of 6 MPa holds 25 kg of liquefied substance. In gaseous form, after evaporation, the liquid is transformed into 12.5 thousand liters of gas.
Dimensions of methane cylinders of the fourth type
Model: TK-FUJIKIN (TKF), Korea
| Volume (l) | Outer diameter (mm) | Length (mm) | Weight, kg) |
| 42 | 310 | 810 | 16 |
| 61 | 336 | 1000 | 22 |
| 68 | 392 | 850 | 27,1 |
| 73 | 392 | 900 | 28,5 |
| 101 | 452 | 950 | 37,5 |
| 103 | 452 | 950 | 37,5 |
| 107 | 448 | 940 | 40 |
| 123 | 452 | 1110 | 43 |
| 131 | 460 | 1090 | 40 |
| 138 | 358 | 1830 | 47,8 |
| 145 | 485 | 1090 | 46 |
| 164 | 392 | 1820 | 61 |
| 230 | 458 | 1830 | 63 |
| 35 | 280 | 864 | 16 |
| 91 | 452 | 850 | 39,5 |
| 99 | 462 | 864 | 35 |
| 103 | 452 | 950 | 43 |
| 105 | 408 | 1143 | 38 |
| 132 | 408 | 1397 | 48 |
| 138 | 462 | 1143 | 45 |
| 175 | 408 | 1829 | 65 |
| 187 | 537 | 1143 | 64 |
| 192 | 408 | 2032 | 66 |
| 194 | 462 | 1524 | 59 |
| 198 | 408 | 2108 | 66 |
| 264 | 537 | 1524 | 82 |
Methane cylinders for passenger cars
The first thing I would like to note is that you should not save on a methane cylinder. If you are going to convert your car to methane fuel, be sure to buy a new cylinder .
Remember! methane is stored and operates under high pressure, which means the tank under it must be durable and free of visible damage and signs of corrosion.
It is mandatory to have the cylinder inspected once every 3-5 years.
Read also: How to polish scratched plastic
For a passenger car, you can choose any of the existing types of gas storage. The author of the article, for his personal car, would prefer type 1. Although it is somewhat heavier, it has a decent service life and technical characteristics that exceed other modern types. The new tank will last at least 10 years without visible corrosion processes.
We would also like to draw your attention to the fact that after 55 explosions of composite gas cylinders of type 2-4 in Uzbekistan, these types were prohibited for use. Currently, the only one allowed in the country is type 1.
In Russia, such bans on use have not yet been introduced. But it is worth noting that the vast majority of cars that suffered from a natural gas explosion were equipped with composite gas cylinders.
Dimensions of methane cylinders of the second type
Model: CNG-2 DIGITRONIC Light
| 27 | 229x855 | 180-200 |
| 38 | 273x850 | 220-240 |
| 55 | 316x910 | 270-300 |
| Volume (liters) | Size(mm) | Weight, kg) |
| 50 | 325 x 780 | 40 |
| 55 | 325 x 830 | 44 |
| 60 | 325 x 880 | 47 |
| 65 | 356 x 800 | 57 |
| 70 | 356 x 880 | 60 |
| 80 | 356 x 980 | 65 |
| 80 | 406 x 800 | 64 |
| 90 | 406 x 890 | 72 |
| 100 | 406 x 980 | 79 |
| 110 | 406 x 1140 | 91 |
| 120 | 406 x 1180 | 98 |
Read also: Shank nut KAMAZ 6520
Measurement with a special device
Modern manufacturers offer users equipment whose readings do not depend on external and internal factors. The measuring principle is based on ultrasound. The device is placed next to the cylinder and the degree of fullness is determined by the color indicator. By moving along the wall of the container from top to bottom, you can determine to what level it is still filled. The device is expensive, but it is very useful for the constant use of technical gases. Measurements are carried out quickly without additional manipulations with the cylinder and calculations.
Similar articles
- Types and areas of use of heat guns
- What types of car gas cylinders are there?
- Is inspection and testing of gas cylinders necessary?
Weighing
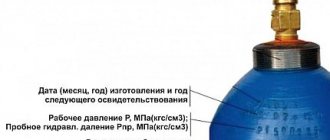
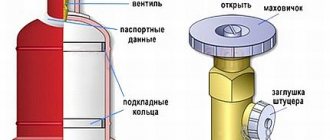
The simplest method, accessible to every consumer and not requiring complex mathematical calculations. Before refilling a gas cylinder in Moscow or any other city, you need to study the container markings located on the bottom. The inscription must contain the manufacturer's data, the date of the last certification, the date of production, the nominal volume, dimensions, operating pressure and weight of the empty container. After this, all that remains is to weigh the cylinder with the remaining gas and determine the difference.
To roughly calculate the amount of content, you can multiply the resulting value by 2. This approximation is acceptable, since the weight of compressed household gas is approximately 0.5 kg/l. That is, if the difference in mass was 1 kg, then the amount of residue is about 2 liters. Knowing the remaining gas will help you calculate when to replace the cylinder.
Why is this necessary?
According to the rules for operating gas cylinders, the gas contained in them cannot be completely consumed. The residual gas pressure inside the cylinder must be at least 0.5 kgf/cm2 (0.05 MPa), otherwise the emptied container will not be accepted for refilling. This requirement to control the gas balance prevents the possibility of foreign substances from the external environment entering the cylinder. If the contents are completely used up, an additional washing operation must be carried out. After this, the cylinders are refilled or stored in a warehouse in an empty state with safety caps. What methods are used to determine the remaining amount of gas?
Checking the amount of gas in the car tank
Without using special equipment, the remaining gas in a car can only be determined by mileage. The tank is fully refilled, then the speedometer is set to zero. While driving, you should only use gas until it runs out. The distance is remembered, which will help you find out the fuel consumption. This value will be taken into account on subsequent trips.
This method is not very accurate and not very convenient, since its value is influenced by the conscientiousness of the workers at the gas station, weather conditions when driving, speed and frequency of stops. If you need the most accurate calculation of the gas remaining in the cylinder, it is better to use special technical sensors.
Source drive2.ru
When is gas tank certification performed?
The frequency of technical certification for vessels operating under excess pressure is 5 years. That is, from the date of manufacture, every 5 years the cylinder must be subjected to tests, during which the integrity of the body and valve, the weight of the structure, the internal capacity and the ability to withstand increased pressure are determined.
However, in some situations, the survey is performed earlier than the deadline, when:
- valve is broken;
- a leak was detected at the cylinder-valve connection;
- the ring on the neck is faulty or missing;
- shoe damaged;
- The painting of the outer surface is of poor quality.
The decision to repair or discard such vessels is made only based on the results of a visual inspection and technical studies.
Water for volume determination
If you need to quickly determine the volume of gas at home or while traveling, use a simple calculation method using ordinary water. To do this you need to prepare:
- 1 partially used tank;
- 1 empty tank of appropriate volume;
- 1 full cylinder with the same volume;
- Marker for marks;
- A large saucepan in which to place the tank;
- Ordinary water.
Source drive2.ru
Then do the following:
- Pour water into the pan until it reaches about halfway;
- Place the tank in the water in a vertical direction;
- Note the level of contact with the liquid;
- Remove the cylinder and mark the waterline with a marker;
- Transfer labels to all tanks;
- Immerse the partially used cylinder in water;
- Note the location of the waterline and mark it with a marker;
- Compare the marks on all three cylinders.
How to properly clean a gas cylinder?
Clear
a used
cylinder
can be removed from accumulated residues with a solution of: acetic acid;
potassium permanganate; detergents and soaps. ... When preparing a container for cleaning, you must:
- unscrew rather than cut off the valve;
- don't hit;
- rinse away from fire and living quarters;
- Do not cut with a grinder, but only with a hacksaw.
Interesting materials:
How much does it cost to take Ielts in Kazakhstan? How much does it cost to make a deed of gift in Kazakhstan? How much does it cost to get a patent in Moscow? How much does it cost to get a passport in Ukraine 2022? How much does a donation transaction cost at a notary? How much does the Northern Commission cost? How much does SMS bank cost? How much does it cost to remove and install a suspended ceiling? How much does it cost to deregister a car in St. Petersburg? How much does it cost to deregister?