Lessons archive › Chemistry 8th grade
In lesson 18 “ Physical and chemical properties of oxygen ” from the course “ Chemistry for Dummies ” we will find out what physical and chemical properties oxygen has and learn about combustion reactions.
Like any chemical substance, oxygen has its own set of physical and chemical properties by which it can be distinguished from other substances.
General information
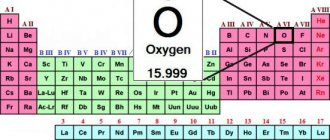
Oxygen is a gaseous chemical element. It is designated by the symbol O, has an atomic number of 8 and an atomic weight of 15.9994, and its molar mass is 32 g/mol. Oxygen formula O2. The diagram of the electronic configuration of the oxygen atom is 1s 2 2s 2 2p 4. The structure of the oxygen atom has two shells, like all elements located in the second period.
It is of great interest because it is an essential element in the respiratory processes of most living cells and combustion processes. It is the most common element in the earth's crust. Almost one-fifth (by volume) of air is O2. Oxidation state -2.
Under normal conditions, O2 is a colorless, odorless and tasteless gas. It condenses into a light blue liquid. It is a reactive element that forms oxides with all other elements except helium, neon, argon and krypton. It is moderately soluble in water (30 cm3 per 1 liter of dissolving water) at a temperature of 20 degrees Celsius.
Test on the topic
- /5
Question 1 of 5How many electrons does an oxygen atom have?
Start test
Hall of Fame
To get here, take the test.
- Alexander Kotkov
5/5
- Mila Kondratieva
5/5
- Semyon Goldfarb
5/5
- Alexander Kotkov
5/5
History of discovery
What is air? Ancient peoples thought deeply about this issue. And this is not surprising when you realize how important air is for many processes. Objects cannot burn without air. Man cannot survive without air. In fact, ancient peoples thought that air was supposed to be an "element." But they used the word “element” in a slightly different meaning than modern scientists. For ancient people, the element was something very important and basic. Air fits this description, along with fire, water and earth. Subsequently, many scientists influenced the discovery of such an element as oxygen:
- The first person in Western Europe to describe the “parts” of air was the Italian artist and scientist Leonardo da Vinci (1452−1519). Leonardo noted that air is not completely consumed when something is burned in it. Therefore, he said that air must consist of two parts: one part that is spent on combustion, and one part that does not participate in the process. For many years, Leonardo's ideas were not very popular among scientists. The problem was that early chemists did not have good equipment like modern laboratories. They found it difficult to collect air samples and then study it.
- In the early 1700s, chemists began to learn more about air, but in a somewhat roundabout way. For example, in 1771 and 1772, Karl Wilhelm Scheele studied the effect of heat on a number of compounds. In one experiment, he used silver carbonate (Ag 2 CO 3), mercuric carbonate (HgCO 3) and magnesium nitrate (Mg(NO 3) 2). When he heated these compounds, he found that gas was released. He then studied the properties of this gas and found that the flame burned brightly in it. He also noticed that animals could stay in it without pain. Without knowing this, Scheele discovered oxygen.
- About two years later, Joseph Priestley conducted similar experiments by heating mercuric oxide (HgO) in a flame. The compound disintegrated, resulting in the formation of liquid metallic mercury and gas. When Priestley tested the new gas, he found the same properties and characteristics as Scheele. Priestley even tried to inhale the gas that he managed to obtain.
- Antoine-Laurent Lavoisier (1743−94) is often called the father of modern chemistry. He received this title for a number of reasons. The most important of these is the explanation he discovered for the process of combustion (burning). Before Lavoisier's research, chemists believed that a burning object released a substance into the air. They called this substance phlogiston. For example, when a tree burned, chemists said that phlogiston moved from the tree into the air. Lavoisier showed that this idea was incorrect. When something burns, it is actually reacting with oxygen in the air. Combustion, Lavoisier said, is simply oxidation (the process by which a substance combines with O2). This discovery gave chemists a completely new way of looking at chemical changes. The phlogiston theory gradually began to die out. Many ideas began to develop that are used today in modern chemistry.
Some people think that Scheele should be given credit for the discovery of oxygen. He completed his experiments before Priestley. But his publisher was very slow in printing the scientist’s reports. They actually came out after Priestley's reports. Thus, most historians agree that Scheele and Priestley should share the right to discover oxygen.
Neither Scheele nor Priestley fully understood the importance of their discovery. This step was taken by the French chemist Antoine-Laurent Lavoisier (1743−94). Lavoisier was the first person to declare that the new gas was an element. He was also the first person to explain how oxygen is involved in combustion. In addition, he suggested a name for the gas.
The word oxygenium (“oxygen”) comes from Greek words meaning “acid-producing.” Lavoisier chose this name because he thought that all acids contain O2. Therefore, the new element was responsible for the “formation of acids.” However, in this respect Lavoisier was mistaken. Not all acids contain oxygen.
Molecule structure
The oxygen molecule consists of two atoms, the chemical formula is O2. How is an oxygen molecule formed? The mechanism of its formation is covalent nonpolar, in other words, due to the sharing of an electron with each atom. The bond between oxygen molecules is also covalent and non-polar, while it is double, because each of the oxygen atoms has two unpaired electrons at the outer level.
This is what an oxygen molecule looks like; due to its characteristics, it is very stable. Many chemical reactions involving it require special conditions: heating, high pressure, and the use of catalysts.
Element in the environment
The Earth's crust is composed primarily of silicon-oxygen minerals, and many other elements are present as their oxides. Oxygen gas makes up one fifth of the atmosphere. O2 in the Earth's atmosphere is produced by photosynthesis by plants , and accumulated over long periods of time as they used the abundant supplies of carbon dioxide in the early atmosphere and released oxygen.
The element is highly soluble in water, which makes life possible in rivers, lakes and oceans. The water in these bodies of water must be regularly supplied with oxygen because when its supply of O2 is depleted, it can no longer support fish and other aquatic organisms.
Almost all chemicals, except noble gases, combine with oxygen to form compounds. Water, H2O, and silica, SiO2, the main component of sand, are among the most common double oxygen compounds. Among compounds that contain more than two elements, the most common are silicates, which form most rocks and soils. Other compounds that occur in abundance in nature are calcium carbonate (limestone and marble), calcium sulfate (gypsum), aluminum oxide (bauxite) and various iron oxides, which are used as a metal source.
The element is found in all types of minerals. Some common examples include oxides, carbonates, nitrates, sulfates and phosphates. Oxides are chemical compounds that contain oxygen and another element. Carbonates are compounds that contain oxygen, carbon and one other element. An example is sodium carbonate or soda, soda ash or salt soda (Na2CO3), which is often found in detergents and cleaning products.
Nitrates, sulfates and phosphates also contain oxygen. Other elements in these compounds are nitrogen, sulfur or phosphorus plus one other element. Examples of these compounds are potassium nitrate or saltpeter (KNO3), magnesium sulfate or Epsom salts (MgSO4) and calcium phosphate (Ca3(PO4)2).
Receipt
The most common and easiest source of oxygen is air. Moreover, it is inexhaustible and is present everywhere in our lives. To obtain the necessary substances from it, it is liquefied and then divided into liquid nitrogen and oxygen.
Another way to obtain liquid is by condensing it from gas. To do this, simply lower a copper coil into a container of liquid nitrogen, and then pass oxygen gas through the coil. The temperature of nitrogen is lower than that of oxygen, so as it passes through the copper tube, the gas will condense and turn into a liquid. In this case, a small layer of snow forms on the surface of the coil.
Isotopes of oxygen
There are three naturally occurring isotopes of O2 : oxygen-16, oxygen-17 and oxygen-18. Isotopes are two or more forms of an element. They differ from each other by their mass number. The number written to the right of the element's name is the mass number. It represents the number of protons plus neutrons in the nucleus of an element's atom. The number of protons defines an element, but the number of neutrons in an atom of any one element can vary. Each variation is an isotope.
There are also five known radioactive isotopes of the element. A radioactive isotope is one that breaks apart and emits some form of radiation. Radioactive isotopes are formed when very small particles burn atoms. These particles stick to atoms and make them radioactive.
Health effects
Oxygen is essential to all life forms as it is a constituent of DNA and almost all other biologically important compounds. In the lungs, this element is absorbed by the iron atom in the center of hemoglobin in the blood and is thus transported to where it is needed.
Every person needs this element to breathe, but, as with many things, too much of it is harmful. If a person is exposed to large amounts of O2 for a long time, lung damage can occur. Inhaling 50-100% oxygen at normal pressure over an extended period causes lung damage. People who work with frequent or potentially high exposure to pure elements should undergo pulmonary function tests before starting work and upon completion.
Storage and precautions
Liquid oxygen does not ignite or explode on its own, it is not toxic to humans and is not harmful to the environment. However, the active reaction in chemical processes, as well as the cryogenic effect, make it not an entirely safe substance.
When working with it, you need to keep lubricants, combustible and flammable materials away, and always use gloves and protective clothing. Oxygen at very low temperatures easily damages the skin and can lead to frostbite, injury and death of living cells. If the fluid covers a significant part of the body, it can even end in death.
Technical and medical liquid oxygen is stored in Dewar vessels, which are made primarily of steel or aluminum. These are cylindrical containers with double walls, between the walls of which there is a vacuum cavity, as well as thermal insulation materials. They work on the principle of thermoses, keeping liquids inside well.
Chemical properties
At standard temperature and pressure, two atoms of an element bond to form oxygen dioxide, a colorless, odorless, and tasteless diatomic gas with the formula O2. This element is a member of the chalcogen group of the periodic table and is a highly reactive non-metallic element. It easily forms compounds (particularly oxides) with almost all other elements.
Oxygen is a strong oxidizing agent and has the second highest electronegativity of all reactive elements, second only to fluorine. By mass, oxygen is the third most abundant element in the universe after hydrogen and helium and the most abundant element by mass in the Earth's crust, accounting for almost half the mass of the Earth's crust. Free oxygen is chemically reactive to appear on Earth without the photosynthetic action of living organisms, which use the energy of sunlight to produce elemental oxygen from water.
Elemental O2 began to accumulate in the atmosphere only after the evolutionary emergence of photosynthetic organisms, approximately 2.5 billion years ago. Diatomic oxygen gas currently makes up 20.8% of air volume.
The most important chemical properties of oxygen are:
- Maintaining combustion. It helps other objects burn. Burning charcoal is an example. Charcoal is almost pure carbon.
- Connects to elements at room temperature. Rust is an example. Rust is a process by which metal combines with O2.
- Reacts with many compounds. Decay is the process by which once living matter combines with oxygen. The breakdown products are mainly carbon dioxide (CO2) and water (H2O):
O2 itself does not burn. A lit match in a cylinder with a pure element burns much brighter, but the oxygen does not ignite.
Physical properties
Oxygen is more soluble in water than nitrogen. Water contains approximately one O2 molecule for every two N2 molecules, compared to an atmospheric ratio of approximately one to four. The density of oxygen in the gaseous state is 1.42897 kg/m3. Other physical properties of the element are:
- The gas in its normal state is colorless, odorless and tasteless. Liquid oxygen is slightly paramagnetic. It is reactive and forms oxides with all elements except helium, neon, krypton and argon. Moderately soluble in water.
- The element condenses at a temperature of -182.95C and freezes at -218.79C. In both liquid and solid states it is a transparent substance of light blue color.
- The existence of an element in two allotropic forms. Allotropes are modifications of an element with different physical and chemical properties. Allotropes of O2 are diatomic oxygen and ozone, or triatomic. Ozone occurs in fairly large quantities under special conditions. For example, unusually large amounts of ozone are found in the Earth's upper atmosphere. This ozone layer is important for life on the planet. It protects against harmful radiation that comes from the Sun. Ozone at ground level is not useful for life and can cause health problems in plants, people and animals.
- Atomic oxygen is very highly reactive. It is capable of oxidizing atoms of elements that are unusual for this organism, and is one of the strongest antioxidants that eliminates oxygen starvation of tissues and destroys almost any pathogenic microflora (fungi, viruses, bacteria and others) and excess free radicals.
Application of oxygen
Molecular dioxide O2 is essential for cellular respiration in all aerobic organisms. Its reactive species, such as superoxide ion (O2-) and hydrogen peroxide (H2O2), are dangerous byproducts of oxygen use in organisms. However, parts of the immune system of higher organisms use reactive peroxide, superoxide, and singlet oxygen to destroy invading microbes. Reactive species also play an important role in the hypersensitive response of plants to pathogenic microorganisms.
At rest, an adult inhales between 1.8 and 2.4 g of oxygen per minute. This amounts to more than 6 billion tons of the element inhaled by humanity per year. Areas of use include the following:
- People who have breathing problems use oxygen masks and tanks to get the oxygen they need.
- It is used in rocket fuel, combined with hydrogen in the engine. When hydrogen and oxygen combine, they release very large amounts of energy. The energy is used to launch a rocket into space.
- Metal production accounts for the largest percentage of O2 use. For example, the element is used to burn carbon and other impurities found in iron to produce steel. A small amount of these impurities can be beneficial to the steel, but too much makes it brittle and unusable. Carbon and other impurities are burned off during steel production by blowing O2 through molten iron.
- Used in the production of metals such as copper, lead and zinc. These metals occur in the earth in the form of sulfides, such as copper sulfide (CuS), lead sulfide (PbS) and zinc sulfide (ZnS). The first step in extracting these metals is to convert them into oxides. The oxides are then heated with carbon to produce pure metals.
- It is used in the chemical industry as a starting material for the production of some very important compounds. Sometimes the stages of transition from oxygen to the final compound are lengthy. For example, ethylene gas (C2H4) can be treated with oxygen to form ethylene oxide (CH2CH2O). About 60% of the resulting ethylene oxide is converted to ethylene glycol (CH2CH2 (OH)2). Ethylene glycol is used as an antifreeze and serves as a starting point in the production of polyester fibers, films, plastic containers, bags and packaging materials
- Used in oxyacetylene welding, as an oxidizer for rocket fuel, and in the production of methanol and ethylene oxide.
- Plants and animals use it for respiration.
- Pure oxygen is often used to help patients with respiratory problems breathe easier.
Oxygen and its compounds play a key role in many important processes in life and industry.