Homogenization annealing of aluminum
Ingots are subjected to this type of annealing before pressure treatment to eliminate dendritic segregation, which leads to the formation of a heterogeneous solid solution and the precipitation of brittle nonequilibrium eutectic inclusions CuAl2, Mg2Si, Al2CuMg (S-phase), Al6CuMg4 (T-phase) along grain boundaries and between the branches of dendrites. phase), etc. In the process of homogenization, the composition of solid solution crystallites is leveled, and intermetallic compounds are dissolved. During subsequent cooling, intermetallic compounds are released in the form of evenly distributed small secondary inclusions (Fig. 159, b). As a result, the ductility of the cast alloy increases, which makes it possible to increase the degree of compression during hot forming, the pressing speed and reduce technological waste. Homogenization promotes a fine grain structure in the annealed sheets and reduces the susceptibility to stress corrosion. The homogenization temperature lies slightly below the nonequilibrium solidus and for the most common alloys is 480–530°C. The exposure ranges from 6 to 36 hours. Cooling is carried out in air or together with a furnace.
Recrystallization annealing of aluminum
Recrystallization annealing involves heating a deformed alloy to temperatures above the temperature at which the primary recrystallization ends; it is used to remove hardening and obtain fine grains. For most aluminum alloys, at a degree of deformation of 50–70%, the temperature of the onset of recrystallization is in the range of 280–300°C. The temperature of recrystallization annealing, depending on the composition of the alloy, ranges from 300 to 500 °C (high annealing), with a holding time of 0.5–3.0 hours. After recrystallization annealing of alloys that cannot be strengthened by heat treatment, the cooling rate is chosen arbitrarily. For alloys hardened by heat treatment, the cooling rate to 200–250°C should be
30°C/h. Annealing is used as an intermediate operation between hot and cold deformation. A type of recrystallization annealing is incomplete annealing (low annealing), which allows you to obtain intermediate properties - between the cold-worked state and the recrystallized state. In this case, the annealing temperature must be lower than the temperature at which recrystallization ends to obtain a polygonized structure or a partially recrystallized one, when the hardening is partially removed (annealing temperature 150–300°C). Incomplete annealing is more often used for deformed alloys that cannot be strengthened by hardening and aging.
Annealing methods for aluminum sheets
Annealing of aluminum alloys is not mandatory. But in some cases, without this heat treatment method it is impossible to achieve the desired characteristics of the material.
The reason for the use of annealing may be the special state of the alloy, which can be expressed in a decrease in the ductility of the material.
The use of annealing is recommended when observing three types of conditions:
- The non-equilibrium state characteristic of cast products is associated with the difference in temperature conditions. The cooling rate of cast products significantly exceeds the recommended one, at which the effect of equilibrium crystallization is achieved.
- Plastic deformation. This condition may be caused by technological requirements for the characteristics and shape of the finished product.
- Heterogeneous structure of the material caused by other heat treatment methods, including hardening and aging. In this case, one of the alloying components separates into the intermetallic phase, accompanied by supersaturation of the components.
The above problems can be eliminated by annealing. Normalization of the structure and condition of the aluminum alloy is accompanied by an increase in ductility. Depending on the type of nonequilibrium state, various annealing methods are selected.
Today there are three annealing modes:
- Homogenization. Designed for processing cast ingots. During the heat treatment of ingots at high temperatures, a uniform structure is achieved. This simplifies the rental process while reducing production costs. In some cases it can be used to improve the quality of deformed products. The annealing temperature is maintained within 500 degrees, followed by holding. Cooling can be done in several ways.
- Recrystallization. Used to restore deformed parts. This requires pre-treatment with a press. The annealing temperature varies in the range from 350 to 500 degrees. The holding time does not exceed 2 hours. The speed and method of cooling has no special limits.
- Heterogenization. Additional annealing after other heat treatment methods. This method is necessary for softening aluminum alloys. This processing method makes it possible to reduce the degree of strength while simultaneously increasing the level of ductility. Annealing is carried out at approximately 400 degrees Celsius. Exposure is usually 1-2 hours. This type of annealing significantly improves the performance characteristics of the metal and increases the degree of corrosion resistance.
Annealing of aluminum for softening of aluminum alloys
Annealing to soften the alloys (full annealing) is carried out at 350-430°C with a holding time of 1-2 hours. At these temperatures, complete decomposition of the supersaturated solid solution and coagulation of strengthening phases occurs. To avoid hardening, the cooling rate should not exceed 30°C/h. After annealing, the alloy has low tensile strength values, satisfactory ductility and high resistance to stress corrosion. The annealed material is capable of withstanding cold working with high degrees of deformation.
Aluminum - properties of aluminum, GOSTs, rolled aluminum.
Heat treatment of aluminum alloys: types and modes – Turner
18.12.2019
Heat treatment of aluminum profiles is used to modify the properties of the aluminum alloys from which they are made by changing their microstructure.
The main strengthening mechanisms in aluminum alloys are strengthening due to solid solution alloying and strengthening due to precipitation of secondary phases.
As a rule, one of these mechanisms is dominant in the alloy.
Solid solution of aluminum alloys
A solid solution is obtained by heating an aluminum alloy, during which all phases present in it dissolve to form one homogeneous phase - aluminum with alloying elements dissolved in it. With increasing temperature, the solubility of elements increases, and with decreasing temperature, it decreases.
The mechanism of hardening is that when the aluminum alloy is cooled quickly enough, the dissolved elements remain in the atomic lattice of aluminum and distort and elastically deform it.
This distorted atomic lattice hinders the movement of dislocations and, consequently, plastic deformation of the alloy and thereby increases its mechanical strength.
Aging of aluminum alloys
Aluminum alloys that are hardened by age contain a certain amount of soluble alloying elements, such as some combinations of copper, magnesium, silicon, manganese and zinc.
When subjected to appropriate heat treatment, these dissolved atoms combine into very small particles that are released within the grains of the aluminum alloy. This process is called aging, as it occurs “by itself” at room temperature.
To speed up and achieve greater efficiency in strengthening the aluminum alloy, aging is carried out at elevated temperatures, say 200 °C.
Hardening of aluminum profiles on a press
Press hardening is a very cost-effective technology for heat treatment of aluminum profiles compared to hardening with separate heating. When hardening in a press, aluminum profiles are cooled based on the temperature at which they leave the matrix.
A necessary condition for hardening on a press is that the heating temperature range of the aluminum alloy for hardening must coincide with the temperature range of aluminum profiles at the outlet of the press.
This, in principle, is carried out only for “soft” and “semi-hard” aluminum alloys - technical aluminum, aluminum alloys of the 3xxx and 6xxx series, as well as low-alloy alloys of the 5xxx series (with magnesium up to 3%) and some aluminum alloys of the 7xxx series without copper alloying (7020, 7005 (our 1915), 7003).
The hardening effect for 3xxx and 5xxx aluminum alloys is very small and, as a rule, is not taken into account.
The final mechanical properties of aluminum alloys 3xxx and 5xxx are obtained not as a result of thermal hardening, but during subsequent cold hardening, which may include heat treatment operations: one or more annealing. The strengthening phase for alloys of the 6xxx series is the Mg2Si compound. For more details, see Hardening aluminum profiles on a press
Press hardening of alloys AD31, 6060 and 6063
All aluminum alloys of the 6xxx series can be hardened directly on the press. To fix the dissolved phases in the aluminum solid solution, it is necessary to cool the aluminum profiles at the outlet of the press at a speed not lower than a certain critical speed.
This speed depends on the chemical composition of the aluminum alloy. Typically, enhanced cooling by fans is sufficient for most aluminum profiles, but sometimes it is necessary to cool them with water or a mixture of air and water.
Successful hardening of 6xxx series aluminum alloys depends on the thickness of the profile, as well as the type of alloy and its chemical composition.
In the case of overly massive aluminum profiles, for example, from alloy AD33 (6061) and a relatively slow pressing speed, the material exiting the matrix may not reach the temperature range required for hardening and some of the Mg2Si particles will remain undissolved.
Therefore, with subsequent air, or even water, cooling of the profiles, their complete hardening will not be possible. In such cases, separate heating is used for hardening in special furnaces - usually vertical, followed by cooling in vertical tanks with water. After hardening, aluminum profiles are stretched by 1.5 - 3% to straighten and relieve residual stresses.
Aging of aluminum profiles: artificial and natural
The final operation of heat treatment of aluminum profiles is aging, natural or artificial.
Natural aging occurs naturally over a period of time, which varies for different aluminum alloys - from several weeks to several months. Artificial aging is carried out in special aging ovens.
Typical heat treatment conditions for some 6xxx aluminum alloys are given in Table 1.
Table 1
Heat treatment of aluminum alloys Al-Zn-Mg
Aluminum alloys Al-Zn-Mg without copper alloying (7020, 7005 (1915), 7003) are also classified as “semi-hard” alloys. They are successfully used in the manufacture of car bodies, load-bearing structures, including welded ones.
These aluminum alloys are successfully hardened by aging if the temperature of the profiles at the exit from the press is at least 400 °C. Most often they are used without forced cooling at all due to their tendency to stress corrosion.
At the same time, for example, aluminum alloy 1915 provides a tensile strength of more than 315 MPa even in a hot-pressed state with natural aging of 30 to 35 days.
Hardening of aluminum profiles with separate heating
Aluminum alloys Al–Cu–Mg and Al–Zn–Mg–Cu, as well as alloys of the Al–Mg series with a magnesium content of more than 3% are classified as difficult to press.
Aluminum-magnesium alloys are not subject to thermal hardening, and the process of thermal hardening of aluminum alloys Al–Cu–Mg and Al–Zn–Mg–Cu (2xxx and 7xxx) differs significantly from the heat treatment of 6xxx alloys, which are always hardened in a press.
Hardening of these alloys, for example, alloys 7075 and 2024 (D16), is carried out only with separate heating, most often in vertical furnaces, followed by rapid hardening in vertical baths-tanks with water.
The final heat treatment operation - the aging operation - is carried out either at room temperature (natural aging) or at a given elevated temperature for the required time (artificial aging).
Hardening of hard aluminum alloys
Table 2 presents the strengthening phases of thermally hardenable hard alloys. When heated in a furnace for quenching, they dissolve in a solid solution. The heating process involves holding at a given temperature to achieve a nearly homogeneous solid solution.
The cooling rate of aluminum profiles from the quenching temperature must exceed a certain critical rate, which is different for different aluminum alloys, in order to obtain maximum strength properties and intergranular resistance in the aged state.
For example, for alloy 7075 the cooling rate must be no less than 300 °C/s in the temperature range from 400 to 280 °C. In the quenched state, age-hardened aluminum alloys are unstable.
When aluminum alloys age, submicroscopic particles of the secondary phase are released, which form an irregular dislocation structure. Due to the formation of this structure, the alloy is strengthened.
The size and distribution of these precipitates determines the optimal mechanical properties of the aluminum alloy. Typical heat treatment modes for some hard aluminum alloys are given in Table 3. The heating duration depends on the thickness of the aluminum profiles.
Table 2 Table 3
Sources:1. Saha P.
Heat treatment of aluminum alloys
To harden aluminum alloys, hardening and aging are used. To eliminate nonequilibrium structures and deformation structural defects that reduce the plasticity of the alloy, annealing is used.
Hardening of aluminum alloys
Quenching consists of heating the alloys to a temperature at which excess intermetallic phases are completely or mostly dissolved in aluminum, holding at this temperature and rapidly cooling to room temperature to obtain a supersaturated solid solution. For example, the hardening temperature of alloys of the Al–Cu system (Fig.
1) determined by line abc
, passing above the limiting solubility line for alloys containing less than 5.7% Cu, and below the eutectic line (548 ° C) for alloys containing more Cu.
When alloys containing up to ~ 5% Cu are heated for quenching, the excess CuA12 phase completely dissolves, and upon subsequent rapid cooling, only a supersaturated α-solid solution is fixed, containing as much copper as is present in the alloy (Fig. 2c).
If the content of copper is more than 5% in the structure of the alloys after quenching, there will be a supersaturated α-solid solution of composition corresponding to point b
, and crystals of the CuAl2 compound undissolved during heating. The holding time at the quenching temperature required to dissolve the intermegallide phases depends on the structural state of the alloy, the type of furnace and the thickness of the product.
Sheets, plates, rods, strips with a thickness of 0.5–150 mm can withstand heating in saltpeter baths for 10–80 minutes, and in electric furnaces with forced air circulation, the most widely used for this purpose, for 30–210 minutes. The holding of shaped castings at the hardening temperature is longer (2–15 hours). During this time, coarse precipitates of intermetallic phases dissolve (Fig. 2a).
During hardening, deformed alloys are cooled in cold water, and shaped castings are cooled in heated water (50–100 °C) to avoid warping and cracking. After hardening, the alloys have a relatively low strength σв, σ0.2 and high ductility (delta;, ψ).
| Fig.1. Al–Cu phase diagram |
| Fig.2. Microstructure of aluminum alloys: a – cast alloy Al + 12% Cu (α-solution and eutectic crystals α + CuAl2 and CuAl2); b – cast alloy D16 (α-solution and CuAl2 and Al2MgCu crystals); c – alloy D16 after quenching (α-phase); d – alloy D16 after quenching and aging |
Heat treatment of aluminum and magnesium alloys.
Heat treatment of aluminum and magnesium alloys is a critical operation of the technological process. Its purpose is to change the structure and physical and chemical properties of alloys. The heat treatment mode is selected depending on the alloys and the method of manufacturing blanks and parts from them.
Heat treatment of parts made from aluminum alloys is based on the fact that with decreasing temperature the solubility of many elements in solid aluminum decreases. When heated for quenching, aluminum alloys do not completely crystallize. If the alloy is overheated, resulting in a structure with large grains, then such an alloy is rejected. Therefore, the thermist must be attentive to the heating of parts made of aluminum alloys.
Heat treatment of deformable aluminum alloys. Deformable aluminum alloys are subjected to such types of heat treatment as annealing, hardening, and aging.
Heat treatment of aluminum alloys
Heat treatment of aluminum alloys is designed to adjust the characteristics of the material by exposure to high temperatures.
A wide variety of structures and properties can be achieved by various processing methods. Alloys that contain impurities in the amount of 15-18% have the form of a solid solution. Copper, magnesium, zinc, silicon and other substances are used as additional components, different combinations of which and their percentage directly affect the properties of the material.
In their normal state, aluminum alloys are not very strong, but are quite ductile. The most unstable alloys include a large number of alloying components that affect the equilibrium structure.
Heat treatment methods are used to strengthen aluminum alloys. By uniform heating, which is regulated by technical conditions, the appropriate structure necessary for the initial stage of decomposition of the solid solution is obtained.
Heat treatment can produce many types of material structures that meet production requirements. Heat treatment allows you to create a structure that has no analogues.
Heat treatment of aluminum alloys
To date, many methods have been developed for heat treatment of aluminum products, among which three have gained the most popularity: annealing, hardening, and aging.
Features of heat treatment of aluminum alloys
Aluminum and its alloys require a special approach to heat treatment to achieve a certain strength and structure of the material. Several heat treatment methods are often used. Typically, aging follows after hardening. But some types of materials can be aged without hardening.
This opportunity appears after casting, when the components, at an increased cooling rate, can give the metal the necessary structure and strength. This occurs during casting at a temperature of about 180 degrees. At this temperature, the level of strength and hardness increases, and the degree of ductility decreases.
Each heat treatment method has some features that should be taken into account when processing aluminum products.
Annealing is necessary to impart a uniform structure to the aluminum alloy. Using this method, the composition becomes more homogeneous, the diffusion process is activated and the size of the base particles is equalized. It is also possible to achieve a reduction in the voltage of the crystal lattice. The processing temperature is selected individually, based on the characteristics of the alloy, the required final characteristics and the structure of the material.
Composition and properties of aluminum alloys strengthened by heat treatment
An important step in annealing is cooling, which can be done in several ways. Cooling is usually carried out in an oven or in the open air. Step-by-step combined cooling is also used, first in an oven and then in air.
The characteristics of the finished material directly depend on the rate of temperature decrease. Rapid cooling promotes the formation of supersaturation of the solid solution, and slow cooling promotes a significant level of decomposition of the solid solution.
Quenching is required to strengthen the material by supersaturating the solid solution. This method is based on heating products to temperatures and rapid cooling. This contributes to the complete dissolution of the constituent elements in aluminum. Used for processing wrought aluminum alloys.
To use this method, you need to correctly calculate the processing temperature. The higher the degree, the less time is required for hardening. In this case, it is worth choosing the temperature so that it exceeds the value required for the solubility of the components, but is less than the limit of the metal melt.
The aging method increases the strength of the aluminum alloy. Moreover, it is not necessary to subject the products to artificial aging, since the process of natural aging is possible.
Depending on the type of aging, the rate of structural changes changes. Therefore, artificial aging is more preferable, as it improves productivity. The selection of processing temperature and time depends on the properties of the material and the characteristics of the alloying components.
The right combination of heating level and holding time can increase strength and ductility. This process is called stabilization.
Annealing methods for aluminum sheets
Annealing of aluminum alloys is not mandatory. But in some cases, without this heat treatment method it is impossible to achieve the desired characteristics of the material.
The reason for the use of annealing may be the special state of the alloy, which can be expressed in a decrease in the ductility of the material.
The use of annealing is recommended when observing three types of conditions:
- The non-equilibrium state characteristic of cast products is associated with the difference in temperature conditions. The cooling rate of cast products significantly exceeds the recommended one, at which the effect of equilibrium crystallization is achieved.
- Plastic deformation. This condition may be caused by technological requirements for the characteristics and shape of the finished product.
- Heterogeneous structure of the material caused by other heat treatment methods, including hardening and aging. In this case, one of the alloying components separates into the intermetallic phase, accompanied by supersaturation of the components.
The above problems can be eliminated by annealing. Normalization of the structure and condition of the aluminum alloy is accompanied by an increase in ductility. Depending on the type of nonequilibrium state, various annealing methods are selected.
Today there are three annealing modes:
- Homogenization. Designed for processing cast ingots. During the heat treatment of ingots at high temperatures, a uniform structure is achieved. This simplifies the rental process while reducing production costs. In some cases it can be used to improve the quality of deformed products. The annealing temperature is maintained within 500 degrees, followed by holding. Cooling can be done in several ways.
- Recrystallization. Used to restore deformed parts. This requires pre-treatment with a press. The annealing temperature varies in the range from 350 to 500 degrees. The holding time does not exceed 2 hours. The speed and method of cooling has no special limits.
- Heterogenization. Additional annealing after other heat treatment methods. This method is necessary for softening aluminum alloys. This processing method makes it possible to reduce the degree of strength while simultaneously increasing the level of ductility. Annealing is carried out at approximately 400 degrees Celsius. Exposure is usually 1-2 hours. This type of annealing significantly improves the performance characteristics of the metal and increases the degree of corrosion resistance.
Complete annealing of aluminum and aluminum alloys
After complete annealing, all aluminum alloys - both thermally hardenable and non-thermally hardenable - obtain a state that is the softest, most ductile and most favorable for plastic deformation.
The international designation for this condition is the letter “O”. Sometimes this letter "O" is confused with the number "0".
In domestic standards for aluminum products there is a state of simply “annealing” and this state is designated by the letter “M”. In terms of the meaning and mechanical properties of alloys in this state, this “simple” annealing is precisely complete annealing, as it is understood in international standards.
Technology of annealing sheets of thermally non-hardening aluminum alloys
And annealing of ingots to relieve stress
Annealing of ingots is carried out to eliminate or reduce dendritic segregation of components, relieve residual stresses arising during the casting process and form a structure that provides the best technological properties. In accordance with this, the following types of annealing of ingots are distinguished: a) homogenization; b) to reduce residual stresses; c) heterogenizing. This classification is conditional (since several processes can occur during annealing.
The main parameters of the homogenization annealing mode are temperature and holding time. Heating rates are not significant. The effect of cooling rate is more significant and will be discussed below.
The temperature of homogenization annealing is chosen to be different depending on the composition of the alloy, but close to the temperature of the equilibrium or nonequilibrium solidus.
Holding at the homogenization temperature leads to the dissolution of excess phases and equalization of the chemical composition throughout the volume of the cells. The rate of homogenization depends significantly on the dispersion of nonequilibrium phases. The smaller the dendritic cells and the thinner the particles of nonequilibrium phases, the faster and more complete the dissolution processes occur.
Changes in the structure of the ingot after homogenization have a hereditary effect on the properties of deformed semi-finished products. Plasticity, impact strength, and endurance are significantly increased. The level of strength characteristics depends on the degree of decomposition of the solid solution with the release of aluminum compounds with manganese, chromium, zirconium and other refractory elements with low solubility. If the degree of decomposition is sufficiently high, then the strength characteristics of semi-finished products obtained using high degrees of deformation are somewhat reduced. Homogenization has less influence on the level of strength characteristics of massive pressed semi-finished products.
Ingots are usually cut after homogenization, during which the high thermal stresses inherent in the cast ingot are removed. Sometimes homogenization is not carried out, and stresses must be relieved before cutting. Then the ingots are annealed at temperatures of 275–350 °C for 1–3 hours. This treatment is sufficient to eliminate residual stresses, and the danger of cracking of the ingots during cutting is removed. This temperature range for most aluminum alloys corresponds to the minimum stability of the solid solution. Therefore, during annealing, the supersaturated solutions in the ingot decompose, and the alloys become softer.
General scheme for the production of plates and sheets from aluminum alloys.
The condition of semi-finished products made of aluminum wrought alloys is indicated by numerical and alphabetic markings: M - soft, annealed; T—hardened and naturally aged; T1—hardened and artificially aged; N - hard-worked; 1/4H - quarter hardened; P (or 1/2P)—semi-hard-worked (in contrast to this designation, the letter P included in the alloy grade means that the alloy is wire); HI - intensively cold-coloured (sheet cold-working is approximately 20%); TN—hardened, naturally aged and cold-hardened; Т1Н - hardened, cold-worked and artificially aged; Т1Н1—hardened, cold-hardened by 15-20% and artificially aged.
Technology of annealing sheets of thermally non-hardening aluminum alloys
The only type of heat treatment of aluminum alloys that cannot be strengthened by heat treatment is annealing, and the method of hardening is cold-working.
In relation to the group of alloys under consideration, high and low annealing is used.
High annealing.
High annealing is carried out at elevated temperatures and holding times sufficient for complete softening of the alloys caused by recrystallization. This operation is used as an intermediate heat treatment to remove cold hardening after rolling or as a final heat treatment to obtain semi-finished products with a high level of plastic properties. When assigning high annealing modes, it is necessary to take into account the possible growth of grains (collective recrystallization), which adversely affects the mechanical properties of the alloys.
Low annealing.
During low annealing, which is carried out at relatively low temperatures, polygonization occurs in the metal, and recrystallization does not have time to complete. As is known, the recrystallization process occurs over time, and therefore, at a given annealing temperature, by varying the holding time, it is possible to regulate the degree of work hardening removal from the previous deformation. At low annealing, partial softening and a slight increase in ductility are observed. It is used only as a final heat treatment to meet consumer requirements for the mechanical and physical-chemical properties of semi-finished products. High and low annealing modes for alloys that cannot be strengthened by heat treatment are given in Table 1.
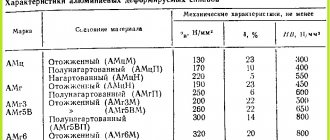
Table 1. Modes of high (numerator) and low (denominator) annealing of sheets of alloys not hardened by heat treatment
| Alloy | tnr, 0С | tozh , 0С | tout, min, with thickness, mm | |
| < 6 | > 6 | |||
| AD00, AD0, AD1, AD | 150-200 | 300-500 150-300 | 2-10 60-180 | 10-30 60-180 |
| AMts | ~300 | 300-500 200-300 | 2-10 60-180 | 10-30 60-180 |
| AM-1, AM-2 | ~300 | 350-420 150-180 | 2-10 60-180 | 10-30 60-180 |
| AM-3 | ~280 | 350-420 250-300 | 2-10 60-180 | 10-30 60-180 |
| AMr4, AMr5, AMr6, AMr6-1-1 | ~280 270-300 | 300-350 310-335 | 30-120 30-120 | 30-180 30-180 |
Sheet heat treatment technology
thermally hardenable alloys
Depending on customer requirements, sheets of thermally hardenable aluminum alloys can be supplied in annealed or hardened and aged states. Some alloys are also supplied in a cold-worked state.
Sheets made of thermally hardenable aluminum alloys are subjected to full or reduced annealing. Alloys of grades D1, D16, D19, VAD1 can also be heated to remove technological hardening.
Complete annealing is called annealing, which ensures a fairly complete occurrence of the processes of decomposition of the solid solution and coagulation of the precipitating phases; Recrystallization also occurs in the cold-worked metal. It is usually carried out at temperatures of 350–430 °C. Upon complete annealing, the material, regardless of the initial state, is completely softened, since the annealing temperature is higher than the temperature at which recrystallization begins. The annealed material is able to withstand cold working with high degrees of deformation. Full annealing can be used as an intermediate and final heat treatment.
When annealing clad semi-finished products, diffusion of alloying elements occurs, most often copper and magnesium, into the cladding layer. In this case, the corrosion resistance of the sheets is significantly reduced, especially if copper diffuses throughout the entire depth of the cladding layer. Therefore, the holding time for complete annealing of clad sheets should be as short as possible.
Some alloys (D1, D16, D19, VAD1, M40, V95 and others) can be supplied hardened by work hardening after hardening or hardening and aging with degrees of deformation of 7-15%. In this case, it is not recommended to use full annealing, since the indicated degrees of deformation may correspond to critical ones, and annealing will cause a sharp grain growth.
Short annealing
is carried out at temperatures that provide the required rate of diffusion and coagulation processes during the decomposition of the solid solution, but at the same time do not lead to heating and subsequent aging when cooled in air. These temperatures are 290–320 °C for the V92ts alloy and 350–370 °C for other wrought alloys. This type of annealing is used to increase the ductility of semi-finished products strengthened by hardening and subsequent aging, as well as to relieve residual stresses. Short annealing is usually applied to semi-finished products and parts, the hardening and aging of which is carried out at machine-building plants. Annealing is carried out after preliminary mechanical processing of semi-finished products to reduce distortion and warping after final machining.
The holding time for reduced annealing is 2-4 hours for all semi-finished products, except clad ones.
Sheets made of thermally hardenable alloys are subjected to hardening and aging. When hardening unannealed sheets, as well as in parts made from them using plastic deformation, upon heating, along with dissolution processes, recrystallization processes occur. The size of the recrystallized grain very much depends on the degree of plastic deformation and the heating rate for quenching. To obtain fine grains, it is necessary to heat at the highest possible speed and avoid critical degrees of deformation during processing before hardening.
Particular attention is paid to the choice of holding time when hardening clad sheets and parts. Due to the diffusion of copper into the cladding layer, a decrease in corrosion resistance and deterioration in the appearance of the sheets is possible. Therefore, the holding time of clad products at the hardening temperature should be minimal.
When loading products into the oven, make sure that they can flow freely from all sides with streams of hot air or saltpeter. Dense packing is not allowed.
The heating temperature for hardening semi-finished products made of aluminum alloys is given in table. 2, exposure time - in table. 3.
After holding at the heating temperature for quenching, the product is transferred to a quenching environment. The time of transfer of sheets from the furnace to the quenching environment significantly affects the mechanical and especially the corrosion properties of sheets after aging.
Table 2. Quenching and aging modes of aluminum alloys.
| steel grade | **Exposure start temperature tnvyd, 0C | tout , 0С | tstar. , 0С | tstar, h |
| AD31, AD33 | 510 | 515-530 | 20 | 240-260 |
| AD35, AB* | 510 | 515-530 | 160-170 | 10-12 |
| AK6*, AK6-1 | 510 | 515-525 | 150-165 | 6-12 |
| AK8* | 490 | 495-505 | 150-165 | 4-12 |
| AK4, AK4-1 | 520 | 525-535 | 190-200 | 7-24 |
| D1 | 490 | 495-510 | 20 | ³96 |
| D16 | 480 | 485-503 | 20 | ³96 |
| D16* (sheets) | 490 | 495-505 | 185-195 | 7-13 |
| D19 | 490 | 495-505 | 185-195 | 12-14 |
| D19 (sheets) | 495 | 500-508 | 185-195 | 12-14 |
| VD17 | 490 | 495-505 | 20 | ³96 |
| VAD1 | 500 | 503-508 | 20 | ³96 |
| M40 | 504 | 504-510 | 20 | ³96 |
| D20 | 525 | 530-540 | 200-220 | 8-12 |
| D21 | 515 | 520-530 | 180-190 | 15-17 |
| VAD23 | 510 | 515-520 | 195-205 | 7-10 |
| V92TS | 445 | 450-470 | 20 | ³72 |
| B93 | 445 | 450-465 | — | — |
| V95, V96, V96TS | 460 | 465-475 | 135-145 | 15-17 |
* Alloys are subjected to artificial and natural aging.
Table 3. Duration of exposure when heating aluminum alloys for hardening in air furnaces (numerator) and saltpeter baths (denominator)
| Type of semi-finished product | l, mm | tout, min |
| Clad sheets | <1,4 1,5-1,9 2,0-4,0 4,1-6,0 6,1-10 | 10-15/5 15-20/7 20-25/10 30-35/15 35-40/20 |
| Unclad sheets, cold-deformed pipes, hot-rolled plates, profiles, rods, strips and hot-pressed bushings | <1,2 1,3-3,0 3,1-5,0 5,1-10 11-20 21-30 31-50 51-75 76-100 101-150 151-200 | 10-20/5 15-30/10 20-45/15 30-60/20 35-75/25 45-90/30 60-120/40 100-150/50 120-180/70 150-210/80 180-240/90 |
| Stampings and forgings | <2,5 2,6-5,0 5,1-15 16-30 31-50 51-75 76-100 101-150 151-200 | 15-30/10 20-45/15 30-50/25 40-60/40 60-150/50 150-210/60 180-240/90-180 210-360/120-240 240-440/180-300 |
Thin sheets cool down more than thick slabs, which have a larger reserve of accumulated heat. Therefore, the transfer time for sheets and slabs up to 50 mm thick is limited to 15 s, and for larger thicknesses to 30 s. For sheets and plates of alloys V95, V96 and V96ts, which are especially prone to corrosion cracking, the transfer time, regardless of thickness, should not exceed 15 s.
The cooling rate during quenching ensures the fixation of the supersaturated solid solution, but it should not be very high in order to avoid severe warping and a high level of residual thermal stresses.
Depending on the value of the critical cooling rate, all aluminum alloys can be divided into the following three groups:
1) with low critical cooling rates - self-hardening alloys, cooled during quenching in air; these include alloys of the A1-Zn-Mg, Al-Mg-Si, Al-Mg-Li systems;
2) with high critical cooling rates; these are alloys of the A1-Cu-Mg, Al-Mg-Si-Cu, Al-Zn-Mg-Cu system;
3) insensitive to changes in cooling rates; these are heat-resistant alloys of the A1-Cu-Mn system with and without the addition of titanium and the AK4-1 alloy of the A1-Cu-Mg-Fe-Ni system.
Running water is usually used as a medium for hardening aluminum alloy sheets. To ensure sufficiently rapid cooling of the sheets, its temperature is maintained within 10–40 °C. The amount of water is chosen so that after immersing the cage and cooling it, the water temperature does not exceed 50 °C.
After hardening, aluminum alloys, strengthened by heat treatment, are subjected to aging. During the aging process, the dimensions of semi-finished products and products change due to volumetric changes during the release of strengthening phases. Therefore, it is necessary to provide for the possibility of free movement of cage products, and mechanical processing should be carried out after aging.
The aging modes of parts and semi-finished products made of aluminum alloys are shown in Table. 2.
Overaging, called softening aging
, leads to structural changes when the coherence of the matrix and metastable intermetallic phases is partially or completely eliminated. This is accompanied by a slight decrease in strength and an increase in fracture toughness and corrosion resistance. Therefore, modes of softening aging have been introduced for some alloys.
The permissible break between hardening and artificial aging, which ensures high mechanical properties, is different for different alloys. It is not limited for alloys AK8, AK4, D16, D19, VAD23 and V92ts. For alloys AD31, ADZZ, AD35 and AB, the break should not exceed 1 hour. For alloys AK6, AK4-1, D1, the permissible break is 6-24 hours.
Aluminum alloys in a freshly quenched state have high ductility comparable to theirs. ductility in the annealed state. Therefore, it is possible to carry out various technological operations associated with plastic deformation of the material. The period of time after quenching during which the alloy retains its ductility depends on the nature of the alloy. With a long holding time, ductility deteriorates due to aging.
The rate of natural aging strongly depends on temperature, even in the range from (-10) to (+ 25) °C. A decrease in temperature by 5 °C reduces the rate of aging by approximately half. Therefore, to maintain high ductility, it is advisable to store alloys at temperatures below room temperature, for example, in refrigerators, before deformation.
And annealing of ingots to relieve stress
Annealing of ingots is carried out to eliminate or reduce dendritic segregation of components, relieve residual stresses arising during the casting process and form a structure that provides the best technological properties. In accordance with this, the following types of annealing of ingots are distinguished: a) homogenization; b) to reduce residual stresses; c) heterogenizing. This classification is conditional (since several processes can occur during annealing.
The main parameters of the homogenization annealing mode are temperature and holding time. Heating rates are not significant. The effect of cooling rate is more significant and will be discussed below.
The temperature of homogenization annealing is chosen to be different depending on the composition of the alloy, but close to the temperature of the equilibrium or nonequilibrium solidus.
Holding at the homogenization temperature leads to the dissolution of excess phases and equalization of the chemical composition throughout the volume of the cells. The rate of homogenization depends significantly on the dispersion of nonequilibrium phases. The smaller the dendritic cells and the thinner the particles of nonequilibrium phases, the faster and more complete the dissolution processes occur.
Changes in the structure of the ingot after homogenization have a hereditary effect on the properties of deformed semi-finished products. Plasticity, impact strength, and endurance are significantly increased. The level of strength characteristics depends on the degree of decomposition of the solid solution with the release of aluminum compounds with manganese, chromium, zirconium and other refractory elements with low solubility. If the degree of decomposition is sufficiently high, then the strength characteristics of semi-finished products obtained using high degrees of deformation are somewhat reduced. Homogenization has less influence on the level of strength characteristics of massive pressed semi-finished products.
Ingots are usually cut after homogenization, during which the high thermal stresses inherent in the cast ingot are removed. Sometimes homogenization is not carried out, and stresses must be relieved before cutting. Then the ingots are annealed at temperatures of 275–350 °C for 1–3 hours. This treatment is sufficient to eliminate residual stresses, and the danger of cracking of the ingots during cutting is removed. This temperature range for most aluminum alloys corresponds to the minimum stability of the solid solution. Therefore, during annealing, the supersaturated solutions in the ingot decompose, and the alloys become softer.
General scheme for the production of plates and sheets from aluminum alloys.
The condition of semi-finished products made of aluminum wrought alloys is indicated by numerical and alphabetic markings: M - soft, annealed; T—hardened and naturally aged; T1—hardened and artificially aged; N - hard-worked; 1/4H - quarter hardened; P (or 1/2P)—semi-hard-worked (in contrast to this designation, the letter P included in the alloy grade means that the alloy is wire); HI - intensively cold-coloured (sheet cold-working is approximately 20%); TN—hardened, naturally aged and cold-hardened; Т1Н - hardened, cold-worked and artificially aged; Т1Н1—hardened, cold-hardened by 15-20% and artificially aged.
Technology of annealing sheets of thermally non-hardening aluminum alloys
The only type of heat treatment of aluminum alloys that cannot be strengthened by heat treatment is annealing, and the method of hardening is cold-working.
In relation to the group of alloys under consideration, high and low annealing is used.
High annealing.
High annealing is carried out at elevated temperatures and holding times sufficient for complete softening of the alloys caused by recrystallization. This operation is used as an intermediate heat treatment to remove cold hardening after rolling or as a final heat treatment to obtain semi-finished products with a high level of plastic properties. When assigning high annealing modes, it is necessary to take into account the possible growth of grains (collective recrystallization), which adversely affects the mechanical properties of the alloys.
Low annealing.
During low annealing, which is carried out at relatively low temperatures, polygonization occurs in the metal, and recrystallization does not have time to complete. As is known, the recrystallization process occurs over time, and therefore, at a given annealing temperature, by varying the holding time, it is possible to regulate the degree of work hardening removal from the previous deformation. At low annealing, partial softening and a slight increase in ductility are observed. It is used only as a final heat treatment to meet consumer requirements for the mechanical and physical-chemical properties of semi-finished products. High and low annealing modes for alloys that cannot be strengthened by heat treatment are given in Table 1.
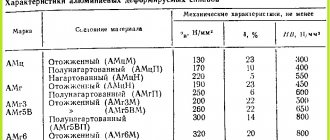
Table 1. Modes of high (numerator) and low (denominator) annealing of sheets of alloys not hardened by heat treatment
| Alloy | tnr, 0С | tozh , 0С | tout, min, with thickness, mm | |
| < 6 | > 6 | |||
| AD00, AD0, AD1, AD | 150-200 | 300-500 150-300 | 2-10 60-180 | 10-30 60-180 |
| AMts | ~300 | 300-500 200-300 | 2-10 60-180 | 10-30 60-180 |
| AM-1, AM-2 | ~300 | 350-420 150-180 | 2-10 60-180 | 10-30 60-180 |
| AM-3 | ~280 | 350-420 250-300 | 2-10 60-180 | 10-30 60-180 |
| AMr4, AMr5, AMr6, AMr6-1-1 | ~280 270-300 | 300-350 310-335 | 30-120 30-120 | 30-180 30-180 |
Sheet heat treatment technology
thermally hardenable alloys
Depending on customer requirements, sheets of thermally hardenable aluminum alloys can be supplied in annealed or hardened and aged states. Some alloys are also supplied in a cold-worked state.
Sheets made of thermally hardenable aluminum alloys are subjected to full or reduced annealing. Alloys of grades D1, D16, D19, VAD1 can also be heated to remove technological hardening.
Complete annealing is called annealing, which ensures a fairly complete occurrence of the processes of decomposition of the solid solution and coagulation of the precipitating phases; Recrystallization also occurs in the cold-worked metal. It is usually carried out at temperatures of 350–430 °C. Upon complete annealing, the material, regardless of the initial state, is completely softened, since the annealing temperature is higher than the temperature at which recrystallization begins. The annealed material is able to withstand cold working with high degrees of deformation. Full annealing can be used as an intermediate and final heat treatment.
When annealing clad semi-finished products, diffusion of alloying elements occurs, most often copper and magnesium, into the cladding layer. In this case, the corrosion resistance of the sheets is significantly reduced, especially if copper diffuses throughout the entire depth of the cladding layer. Therefore, the holding time for complete annealing of clad sheets should be as short as possible.
Some alloys (D1, D16, D19, VAD1, M40, V95 and others) can be supplied hardened by work hardening after hardening or hardening and aging with degrees of deformation of 7-15%. In this case, it is not recommended to use full annealing, since the indicated degrees of deformation may correspond to critical ones, and annealing will cause a sharp grain growth.
Short annealing
is carried out at temperatures that provide the required rate of diffusion and coagulation processes during the decomposition of the solid solution, but at the same time do not lead to heating and subsequent aging when cooled in air. These temperatures are 290–320 °C for the V92ts alloy and 350–370 °C for other wrought alloys. This type of annealing is used to increase the ductility of semi-finished products strengthened by hardening and subsequent aging, as well as to relieve residual stresses. Short annealing is usually applied to semi-finished products and parts, the hardening and aging of which is carried out at machine-building plants. Annealing is carried out after preliminary mechanical processing of semi-finished products to reduce distortion and warping after final machining.
The holding time for reduced annealing is 2-4 hours for all semi-finished products, except clad ones.
Sheets made of thermally hardenable alloys are subjected to hardening and aging. When hardening unannealed sheets, as well as in parts made from them using plastic deformation, upon heating, along with dissolution processes, recrystallization processes occur. The size of the recrystallized grain very much depends on the degree of plastic deformation and the heating rate for quenching. To obtain fine grains, it is necessary to heat at the highest possible speed and avoid critical degrees of deformation during processing before hardening.
Particular attention is paid to the choice of holding time when hardening clad sheets and parts. Due to the diffusion of copper into the cladding layer, a decrease in corrosion resistance and deterioration in the appearance of the sheets is possible. Therefore, the holding time of clad products at the hardening temperature should be minimal.
When loading products into the oven, make sure that they can flow freely from all sides with streams of hot air or saltpeter. Dense packing is not allowed.
The heating temperature for hardening semi-finished products made of aluminum alloys is given in table. 2, exposure time - in table. 3.
After holding at the heating temperature for quenching, the product is transferred to a quenching environment. The time of transfer of sheets from the furnace to the quenching environment significantly affects the mechanical and especially the corrosion properties of sheets after aging.
Table 2. Quenching and aging modes of aluminum alloys.
| steel grade | **Exposure start temperature tnvyd, 0C | tout , 0С | tstar. , 0С | tstar, h |
| AD31, AD33 | 510 | 515-530 | 20 | 240-260 |
| AD35, AB* | 510 | 515-530 | 160-170 | 10-12 |
| AK6*, AK6-1 | 510 | 515-525 | 150-165 | 6-12 |
| AK8* | 490 | 495-505 | 150-165 | 4-12 |
| AK4, AK4-1 | 520 | 525-535 | 190-200 | 7-24 |
| D1 | 490 | 495-510 | 20 | ³96 |
| D16 | 480 | 485-503 | 20 | ³96 |
| D16* (sheets) | 490 | 495-505 | 185-195 | 7-13 |
| D19 | 490 | 495-505 | 185-195 | 12-14 |
| D19 (sheets) | 495 | 500-508 | 185-195 | 12-14 |
| VD17 | 490 | 495-505 | 20 | ³96 |
| VAD1 | 500 | 503-508 | 20 | ³96 |
| M40 | 504 | 504-510 | 20 | ³96 |
| D20 | 525 | 530-540 | 200-220 | 8-12 |
| D21 | 515 | 520-530 | 180-190 | 15-17 |
| VAD23 | 510 | 515-520 | 195-205 | 7-10 |
| V92TS | 445 | 450-470 | 20 | ³72 |
| B93 | 445 | 450-465 | — | — |
| V95, V96, V96TS | 460 | 465-475 | 135-145 | 15-17 |
* Alloys are subjected to artificial and natural aging.
Table 3. Duration of exposure when heating aluminum alloys for hardening in air furnaces (numerator) and saltpeter baths (denominator)
| Type of semi-finished product | l, mm | tout, min |
| Clad sheets | <1,4 1,5-1,9 2,0-4,0 4,1-6,0 6,1-10 | 10-15/5 15-20/7 20-25/10 30-35/15 35-40/20 |
| Unclad sheets, cold-deformed pipes, hot-rolled plates, profiles, rods, strips and hot-pressed bushings | <1,2 1,3-3,0 3,1-5,0 5,1-10 11-20 21-30 31-50 51-75 76-100 101-150 151-200 | 10-20/5 15-30/10 20-45/15 30-60/20 35-75/25 45-90/30 60-120/40 100-150/50 120-180/70 150-210/80 180-240/90 |
| Stampings and forgings | <2,5 2,6-5,0 5,1-15 16-30 31-50 51-75 76-100 101-150 151-200 | 15-30/10 20-45/15 30-50/25 40-60/40 60-150/50 150-210/60 180-240/90-180 210-360/120-240 240-440/180-300 |
Thin sheets cool down more than thick slabs, which have a larger reserve of accumulated heat. Therefore, the transfer time for sheets and slabs up to 50 mm thick is limited to 15 s, and for larger thicknesses to 30 s. For sheets and plates of alloys V95, V96 and V96ts, which are especially prone to corrosion cracking, the transfer time, regardless of thickness, should not exceed 15 s.
The cooling rate during quenching ensures the fixation of the supersaturated solid solution, but it should not be very high in order to avoid severe warping and a high level of residual thermal stresses.
Depending on the value of the critical cooling rate, all aluminum alloys can be divided into the following three groups:
1) with low critical cooling rates - self-hardening alloys, cooled during quenching in air; these include alloys of the A1-Zn-Mg, Al-Mg-Si, Al-Mg-Li systems;
2) with high critical cooling rates; these are alloys of the A1-Cu-Mg, Al-Mg-Si-Cu, Al-Zn-Mg-Cu system;
3) insensitive to changes in cooling rates; these are heat-resistant alloys of the A1-Cu-Mn system with and without the addition of titanium and the AK4-1 alloy of the A1-Cu-Mg-Fe-Ni system.
Running water is usually used as a medium for hardening aluminum alloy sheets. To ensure sufficiently rapid cooling of the sheets, its temperature is maintained within 10–40 °C. The amount of water is chosen so that after immersing the cage and cooling it, the water temperature does not exceed 50 °C.
After hardening, aluminum alloys, strengthened by heat treatment, are subjected to aging. During the aging process, the dimensions of semi-finished products and products change due to volumetric changes during the release of strengthening phases. Therefore, it is necessary to provide for the possibility of free movement of cage products, and mechanical processing should be carried out after aging.
The aging modes of parts and semi-finished products made of aluminum alloys are shown in Table. 2.
Overaging, called softening aging
, leads to structural changes when the coherence of the matrix and metastable intermetallic phases is partially or completely eliminated. This is accompanied by a slight decrease in strength and an increase in fracture toughness and corrosion resistance. Therefore, modes of softening aging have been introduced for some alloys.
The permissible break between hardening and artificial aging, which ensures high mechanical properties, is different for different alloys. It is not limited for alloys AK8, AK4, D16, D19, VAD23 and V92ts. For alloys AD31, ADZZ, AD35 and AB, the break should not exceed 1 hour. For alloys AK6, AK4-1, D1, the permissible break is 6-24 hours.
Aluminum alloys in a freshly quenched state have high ductility comparable to theirs. ductility in the annealed state. Therefore, it is possible to carry out various technological operations associated with plastic deformation of the material. The period of time after quenching during which the alloy retains its ductility depends on the nature of the alloy. With a long holding time, ductility deteriorates due to aging.
The rate of natural aging strongly depends on temperature, even in the range from (-10) to (+ 25) °C. A decrease in temperature by 5 °C reduces the rate of aging by approximately half. Therefore, to maintain high ductility, it is advisable to store alloys at temperatures below room temperature, for example, in refrigerators, before deformation.
What is the purpose of annealing - this is the annealing temperature
If the purpose of annealing is simply to remove strain hardening, then heating to a temperature of about 345 °C will be quite sufficient. If it is necessary to remove strengthening from heat treatment or even simply from cooling from the hot treatment temperature, then special heat treatment is needed to obtain a structure with the release of the strengthening phase in the form of large and separate particles. This heat treatment is complete annealing: holding at temperatures from 415 to 440 °C and slow cooling at a rate of about 30 °C per hour to 260 °C.
High rates of diffusion of alloying elements in aluminum, which are characteristic of such a high temperature, the duration of exposure and slow cooling ensure maximum coalescence (coarsening) of the particles of the strengthening phase, which results in the material - aluminum alloy - minimal hardness.
Aging of aluminum alloys
Aging is carried out to improve the strength characteristics of the product. This type of heat treatment involves exposure to normal temperature conditions.
An increase in strength is achieved by decomposition of the solid solution, which is necessary after hardening, since hardening leads to saturation of the metal.
There are two ways of aging aluminum alloys: natural and artificial.
Natural aging occurs without preheating at normal temperatures. This can happen in an ordinary warehouse or industrial premises, where the air temperature does not exceed 30 degrees.
Natural aging is possible due to a special property of aluminum called the “freshly quenched state.” The properties of products differ significantly immediately after hardening and after some time in storage.
Artificial aging is carried out by heating products to a temperature of 200 degrees. This activates the diffusion process, which promotes improved dissolution of the constituent elements. Exposure ranges from several hours to several days.
It should be noted that artificially aged alloys can be returned to their original state. To do this, you need to heat the product to 250 degrees and hold for up to one minute. Exposure must be carried out in a saltpeter bath at a strictly defined time, accurate to within a few seconds.
Moreover, such a return can be performed several times, without losing the strength of the material, but with a slight change in properties. The return of aged metal is usually carried out in order to restore the ductility necessary to change the shape of the product.
Any type of heat treatment is widely used in industry. Thanks to this, manufacturers have the opportunity to obtain materials that fully meet production requirements. Moreover, such processing of alloys can significantly improve the properties of aluminum and obtain a material that has no analogues.
The main condition for heat treatment is compliance with the requirements and recommendations for the processing temperature and holding time. The slightest deviations can lead to irreversible changes in the properties of the material.
Source
Annealing hold and post-annealing cooling
When annealing, it is important to ensure that the specified temperature is achieved in all parts of the charge and at all points in each product. Therefore, a holding time at the annealing temperature of at least 1 hour is usually prescribed. The maximum annealing temperature is moderately critical: it is recommended not to exceed a temperature of 415 °C due to possible oxidation and grain growth. The heating rate can be critical, for example with alloy 3003, which typically requires rapid heating to prevent grain growth. Relatively slow cooling in still air or a furnace is recommended for all alloys to minimize warping.
Typical full annealing parameters for some aluminum alloys are presented below.
Hardening of aluminum castings
Hardening is not suitable for all types of aluminum alloys. For a successful structural change, the alloy must contain components such as copper, magnesium, zinc, silicon or lithium. It is these substances that are able to completely dissolve in the composition of aluminum, creating a structure that has properties different from aluminum.
This type of heat treatment is carried out under intense heating, allowing the constituent elements to dissolve in the alloy, with further intensive cooling to their normal state.
Thermal transformations in alloys 6060, 6063, AD31
When choosing a temperature regime, you should focus on the amount of copper. Also, you need to take into account the properties of cast products.
In industrial conditions, the heating temperature for hardening ranges from 450 to 560 degrees. Holding products at this temperature ensures the melting of the components in the composition. The holding time depends on the type of product; for deformed ones it usually does not exceed more than an hour, and for cast ones - from several hours to two days.
The cooling rate during hardening must be selected so that the composition of the aluminum alloy is not subject to decomposition. In industrial production, cooling is carried out using water. However, this method is not always optimal, since when thick products are cooled, an uneven temperature decrease occurs in the center and along the edges of the product. Therefore, for large and complex products, other cooling methods are used, which are selected individually.
Full annealing parameters to remove strain hardening
Aluminum alloys
1060, 1100, 1350 3003, 3004, 3105 5005, 5050, 5052, 5083, 5086, 5154, 5182, 5254, 5454, 5456, 5457, 5652 7005 Also used for heat-hardening materials melts, if the purpose of annealing is only to remove strain hardening or partial annealing.
Annealing temperature
Duration of exposure at annealing temperature
About 1 hour. The duration of stay in the furnace should be no longer than necessary to bring all parts of the charge to the annealing temperature.
Cooling after annealing
The cooling rate after annealing is not important.
Annealing temperature
A reduction or complete removal of strain hardening from cold plastic deformation (cold-frettening or cold-working) is achieved by heating to a temperature from 260 to 440 °C. This is true for both thermally hardenable and non-thermally hardenable aluminum alloys.
The rate of softening of cold-worked material strongly depends on temperature. Therefore, the time required to fully anneal a given aluminum alloy with a given degree of work hardening can vary from several hours at low temperatures to several seconds at high temperatures.
Full annealing parameters for removing thermal hardening
Aluminum alloys
2014, 2022, 2024, 2036, 2117, 2124, 2219 6005, 6061, 6060, 6063, 6066 7079, 7050, 7075, 7079, 7178, 7475
Annealing temperature
Duration of exposure at annealing temperature
2 to 3 hours
Cooling after annealing
Cooling at a rate of about 30 °C per hour from the annealing temperature to 260 °C. The rate of subsequent cooling is not important.
Source: Aluminum and Aluminum Alloys, AMS International, 1993.
Cold working: copper, lead and aluminum
Ordinary metals vary greatly in their degree and rate of strain hardening - cold hardening or cold hardening. Copper is hardened quite quickly as a result of cold forging, and, therefore, quickly reduces its malleability and ductility. Therefore, copper requires frequent annealing so that it can be processed further without the risk of destruction.
On the other hand, lead can be hammered into almost any shape without annealing or risk of breaking it. Lead has such a reserve of ductility that allows it to obtain large plastic deformations with a very low degree of strain hardening. However, although copper is harder than lead, it is generally more malleable.
Cold working of iron and steel
Industrial pure iron can be cold worked to large degrees of deformation before it becomes too hard for further processing. Impurities in iron or steel impair the cold workability of the metal to such an extent that most steels cannot be cold worked, except of course special low carbon steels for the automotive industry. At the same time, almost all steel can be successfully processed plastically in a red-hot state.
Why is metal annealing necessary?
The exact nature of the annealing process to which the metal is subjected depends largely on the purpose of the annealed metal. There is a significant difference in the method of annealing between annealing in factories where huge quantities of sheet steel are produced, and annealing in a small auto repair shop, where only one part requires such processing.
In short, cold working is plastic deformation by destruction or distortion of the grain structure of the metal. During annealing, a metal or alloy is heated to a temperature at which recrystallization occurs - the formation of new grains - not deformed and round - instead of old - deformed and elongated - grains. Then the metal is cooled at a given speed. In other words, crystals or grains within the metal that have been displaced or deformed during cold plastic working are given the opportunity to realign and recover to their natural state, but at an elevated annealing temperature.
Annealing of iron and steel
Iron and mild steels must be heated to temperatures of around 900 degrees Celsius and then allowed to cool slowly to ensure they are as "soft" as possible. At the same time, measures are taken to prevent contact of the metal with air in order to avoid oxidation of its surface. When this is done in a small auto repair shop, warm sand is used for this.
High carbon steels require similar processing except that the annealing temperature for them is lower and is about 800 degrees Celsius.
Weldability
One of the main problems with weldability is that a film of oxides very quickly forms on the surface of the metal. Its melting point is more than two thousand degrees, while that of aluminum is much lower. Thus, the film remains on the molten drops, which makes welding very difficult. Because of this, a monolithic seam is not always obtained and the quality of the connection suffers. To combat such a film, additional protection is required, which can be provided by argon welding.
Defects in aluminum welding
When welding aluminum, it is very difficult to control the pool of molten metal, since it has high fluidity. Due to this, it is necessary to use heat-dissipating pads during the welding process. The seam can also be weakened due to the appearance of crystallization cracks, since hydrogen can enter the aluminum and tend to escape, forming stresses and, as a result, cracks. When aluminum is welded, large shrinkage occurs due to the high coefficient of linear expansion. This may cause deformation.
Due to the high thermal conductivity of the metal, to work with it it is necessary to increase the current by approximately 1.5 times, as if working with steel. This is taking into account the fact that the melting point of steel is often much higher. Because of this, thin sheets can be burned through any careless movement. The complexity is also increased by the fact that when welding aluminum at home, it is often impossible to find out exactly what brand is being worked with and what its composition is. This complicates the selection of the electrode.
Annealing aluminum
Aluminum is annealed at a temperature of 350 degrees Celsius. In factories this is done in suitable ovens or salt baths. In the workshop, aluminum is annealed with a gas torch. They say that in this case a wooden splinter is rubbed over the surface of heated metal. When the wood begins to leave black marks, it means that the aluminum has received its annealing. Sometimes a bar of soap is used instead of wood: when the soap begins to leave brown marks, the heating should be stopped. The aluminum is then cooled in water or left to cool in air.