Gas Density Table
Table of densities of liquefied propane-butane mixture (in t/m³) depending on its composition and temperature
| Propane/Butane ratio T, °C | −25 | −20 | −15 | −10 | −5 | 0 | 5 | 10 | 15 | 20 | 25 |
| 100/0 | 0,559 | 0,553 | 0,548 | 0,542 | 0,535 | 0,528 | 0,521 | 0,514 | 0,507 | 0,499 | 0,490 |
| 90/10 | 0,565 | 0,559 | 0,554 | 0,548 | 0,542 | 0,535 | 0,528 | 0,521 | 0,514 | 0,506 | 0,498 |
| 80/20 | 0,571 | 0,565 | 0,561 | 0,555 | 0,548 | 0,541 | 0,535 | 0,528 | 0,521 | 0,514 | 0,505 |
| 70/30 | 0,577 | 0,572 | 0,567 | 0,561 | 0,555 | 0,548 | 0,542 | 0,535 | 0,529 | 0,521 | 0,513 |
| 60/40 | 0,583 | 0,577 | 0,572 | 0,567 | 0,561 | 0,555 | 0,549 | 0,542 | 0,536 | 0,529 | 0,521 |
| 50/50 | 0,589 | 0,584 | 0,579 | 0,574 | 0,568 | 0,564 | 0,556 | 0,549 | 0,543 | 0,536 | 0,529 |
| 40/60 | 0,595 | 0,590 | 0,586 | 0,579 | 0,575 | 0,568 | 0,562 | 0,555 | 0,550 | 0,543 | 0,536 |
| 30/70 | 0,601 | 0,596 | 0,592 | 0,586 | 0,581 | 0,575 | 0,569 | 0,562 | 0,557 | 0,551 | 0,544 |
| 20/80 | 0,607 | 0,603 | 0,598 | 0,592 | 0,588 | 0,582 | 0,576 | 0,569 | 0,565 | 0,558 | 0,552 |
| 10/90 | 0,613 | 0,609 | 0,605 | 0,599 | 0,594 | 0,588 | 0,583 | 0,576 | 0,572 | 0,566 | 0,559 |
| 0/100 | 0,619 | 0,615 | 0,611 | 0,605 | 0,601 | 0,595 | 0,590 | 0,583 | 0,579 | 0,573 | 0,567 |
Distinctive features of liquefied gases:
- high vapor pressure;
- have no smell. To detect leaks in a timely manner, liquefied gases are given a specific odor - odorized with ethylmer-captan (C2H5SH);
- low temperatures and flammability limits. The ignition temperature of butane is 430°C, propane is 504°C. The lower flammability limit of propane is 2.3%, butane is 1.9%;
- propane, butane and their mixtures are heavier than air. In the event of a leak, liquefied gas can accumulate in wells or basements. It is prohibited to install equipment operating on liquefied gas in basement-type premises;
- transition to the liquid phase with increasing pressure or decreasing temperature;
- high calorific value. To burn LPG, a large amount of air is required (to burn 1 m³ of the gas phase of propane, 24 m³ of air is required, and butane - 31 m³ of air);
- large coefficient of volumetric expansion of the liquid phase (the coefficient of volumetric expansion of the liquid phase of propane is 16 times greater than that of water). Cylinders and tanks are filled to no more than 85% of their geometric volume. Filling more than 85% can lead to their rupture, subsequent rapid flow and evaporation of gas, as well as ignition of the mixture with air;
- as a result of evaporation of 1 kg of liquid phase of LPG at n. u. 450 liters of vapor phase is obtained. In other words, 1 m³ of the vapor phase of a propane-butane mixture has a mass of 2.2 kg;
- When 1 kg of propane-butane mixture is burned, about 11.5 kWh of thermal energy is released;
- liquefied gas evaporates intensely and, when it comes into contact with human skin, causes frostbite.
Example:
The density of a propane-butane mixture of 60% propane, 40% butane at an ambient temperature of -20°C will be 0.577 t/m3 or 577 kg/m3
Propane. All aspects of use in everyday life and production of technical propane gas
The current level of technological progress allows people to use a variety of energy sources to solve their everyday problems. One of these resources is technical gas propane. This gas is familiar to absolutely all modern people. Propane is used almost everywhere today. This primarily concerns production processes. Thus, technical gas propane is successfully used for gas-flame work at various production facilities. It is used to perform both metal cutting and welding of non-critical structures. When working with scrap metal, this gas is practically indispensable for the procurement of raw materials. This is due to the fact that other technical gases for welding work are much more expensive and do not allow optimizing production costs.
Propane is also used with no less success in the production of thermal energy. Subsequently, the heat obtained using technical propane gas is used to provide heat supply to both industrial premises and to supply heat to residential complexes.
In everyday life, propane gas finds its use in a variety of areas of human activity. The most common way to use this gas is to use it as an energy carrier for gas stoves and geysers. With its help, a person cooks food and heats water. Also in the individual housing sector, propane is used to organize heating of premises. For this purpose, special equipment is installed. Propane gas is supplied to residential premises using gas pipelines. In some cases, delivery of liquefied propane in special cylinders may also take place.
Recently, propane has found another application in domestic conditions. It is used as automobile fuel. To do this, the vehicle is equipped with a set of equipment that allows the use of this technical gas to operate the engine. This type of optimization leads to significant savings for the motorist. The level of emissions of harmful substances from fuel combustion into the atmosphere is also significantly reduced.
It is worth knowing what propane gas is, what its advantages and disadvantages are. First of all, it is worth noting that technical propane gas is a synthetic product. It is often called natural gas. In part this statement is true. Indeed, propane is produced from natural gas, which is a residual product of the oil extraction process. This is the so-called accompanying gas.
In terms of its properties, propane gas is practically no different from natural gas. It has a high octane number. It also has excellent environmental and performance characteristics. Propane, which is supplied to consumers for use, is in a liquefied state. In order to ensure the liquefied state of gas, it is stored and transported under high pressure. However, you should know that if propane is supplied in cylinders, then the degree of filling of the container should not exceed 90%. This will ensure a high level of safety in the use of propane for domestic and industrial purposes.
Propane gas is not a toxic substance. It is safe enough for use if all technical operation requirements are met. Thus, propane cylinders should not be stored in residential areas. When storing them, ensure that they are not exposed to open heat sources or exposure to sunlight. Gas pipelines must be made of seamless steel pipes.
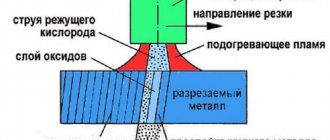
When performing welding work, technical gas propane is used in a mixture with oxygen. This gas successfully replaces acetylene in welding. At the same time, the undoubted advantage of propane is its lower cost and widespread availability. However, there are also disadvantages.
In particular, the combustion temperature of propane is almost a thousand degrees lower than the combustion temperature of acetylene. This creates certain difficulties during welding work. There are areas of work where the use of propane for gas-flame work can significantly reduce the quality of the work performed. Welding metal parts with a thickness greater than six millimeters using propane will be extremely difficult.
The main disadvantages of welding with propane include such side effects as a large percentage of part deformation, the presence of large grains in the weld itself and the heat-affected space. It is quite difficult to adjust the burner torch. In order to correctly set the reducing, oxidizing or normal burner mode for propane use, the welder must have extensive experience in such work.
For welding with technical propane gas, only injection gas torches are used. Today, almost all types of welding equipment torches and cutters offered on the market are injection-based. Therefore, the welder will not have any special problems with choosing suitable welding equipment for metal processing using propane.
The history of the use of propane in everyday life and production goes back an impressive period of time. Propane enters our lives more and more every year. This gas will be used for a long time. This is due to its cost-effectiveness, high consumer properties and accessibility to a wide range of consumers.
What is the difference between butane and propane
- Propane has the chemical formula C3H8, and butane has the chemical formula C4H10. These gases have similar molecular weights and thermal conductivities. The main difference between these alkanes and each other is their boiling point.
- Butane has a boiling point of about -1 degrees Celsius, while propane has a boiling point of -43 degrees Celsius. When these values are reached, butane and propane pass from a gaseous state to a liquid state, i.e. liquefy.
- This leads to different specifics of using these gases.
- Butane is less resistant to low temperatures, so it is difficult to use in the cold season.
- Propane has another significant drawback - it is dangerous to use as a fuel when exposed to high temperatures. When heated, propane expands and presses on the walls of the gas tank or cylinder. This can lead to cracks in the walls of the vessel and to its explosions.
Propane is more explosive because it cannot withstand prolonged exposure to high temperatures.
Mole
All substances are made up of atoms and molecules. In chemistry, it is important to accurately measure the mass of substances that react and are produced as a result. By definition, the mole is the SI unit of quantity of a substance. One mole contains exactly 6.02214076×10²³ elementary particles. This value is numerically equal to Avogadro's constant NA when expressed in units of mol⁻¹ and is called Avogadro's number. Amount of substance (symbol n
) of a system is a measure of the number of structural elements. A structural element can be an atom, molecule, ion, electron, or any particle or group of particles.
Avogadro's constant NA = 6.02214076×10²³ mol⁻¹. Avogadro's number is 6.02214076×10²³.
In other words, a mole is an amount of substance equal in mass to the sum of the atomic masses of atoms and molecules of the substance, multiplied by Avogadro’s number. The unit of quantity of a substance, the mole, is one of the seven basic SI units and is symbolized by the mole. Since the name of the unit and its symbol are the same, it should be noted that the symbol is not declined, unlike the name of the unit, which can be declined according to the usual rules of the Russian language. One mole of pure carbon-12 is equal to exactly 12 g.
How do alkanes differ from other chemical compounds?
“Paraffins” are chemical compounds that have low reactivity. Alkanes do not dissolve in water and do not have any color. When burned, alkanes emit a lot of heat, and their flame is colorless or light blue. This class of compounds is actively used in industry, in particular in the synthesis of fuel and oil.
In the table you can see a list of substances that belong to the class of alkanes.
Propane in industry
The technical form of the substance is used:
- for heating the cabins of heavy-duty vehicles;
- in welding and cutting of various metal structures;
- in perfumery, cosmetic production;
- for the production of varnishes, solvents;
- in the production of printed products at printing plants - it is used to make copy paper and printing ink;
- as a flavoring additive;
- for the production of refrigerant for refrigeration units and air conditioners;
- in the production and coloring of polymers;
- for heating workshops, greenhouses, industrial buildings.
Propane dehydrogenation - a method for producing propylene
Propane dehydrogenation as an industrial method for producing propylene has been used since 1990. There are virtually no by-products during the dehydrogenation process.
In accordance with this technology, propane (and a small amount of hydrogen to reduce coke formation) is fed into a reactor with a fixed or moving catalyst bed at a temperature of 510-700 ºC at atmospheric pressure. The catalyst is platinum supported on activated aluminum oxide containing 20% chromium. With any reactor design, constant regeneration of the catalyst is necessary to maintain its activity. The reactor effluent enters standard separation columns. Unreacted propane and some hydrogen are returned to the process, mixed with a fresh portion of raw materials. The remaining product contains approximately 85% propylene, 4% hydrogen, and light and heavy off-gases.
The use of this technology is justified when the demand for propylene is high, exceeding the demand for ethylene. The absence of by-products eliminates additional efforts to sell them. One of the key points for the production of propylene by dehydrogenation of propane is the difference in prices between propylene and propane. If the difference is not sufficient, it may turn out that the propylene produced will cost more than at market prices. However, it cannot be said that the dehydrogenation process is used only if there is a source of sufficiently cheap propane. In fact, most propane dehydrogenation plants are located in places where there is a special need for propylene, not where cheap propane is available. While most propylene is produced by refining petroleum and its products, producing propylene by dehydrogenating propane produces feedstocks that are not directly tied to oil prices.
The use of propane in the housing and communal services sector
Liquefied gas is used everywhere; it solves many problems in the industrial and domestic spheres. With his help:
- heat residential buildings and production workshops;
- carry out gas-flame work, including cutting, welding of metal structures, pipelines, etc.;
- heat up bitumen when carrying out roofing work;
- power water heating equipment in houses not connected to centralized systems;
- indoor air conditioning systems are functioning.
Would you like a consultation?
Call us on the phone!
+7 Mon.-Fri. from 9:00 to 18:00, lunch from 13:00 to 14:00, Sat. from 9.00 to 15:00
What are propane and butane and how are they obtained?
Propane and butane, like all alkanes, are flammable substances that are used as fuel. However, due to different physical properties, they have different specializations. Propane and butane are homologues of methane.
They can be obtained from natural raw materials - for example, gas, rock wax or oil. In addition, propane and butane can be synthesized from a mixture of carbon monoxide and hydrogen.
In the laboratory, butane and propane are obtained using the catalytic hydrogenation of propene, propine, butene and butine, as well as by the Wurtz reaction.
Transportation and storage
Since evaporation of liquid in LPG occurs even at 0 °C, these gases are usually stored strictly in closed containers.
Large consumers receive hydrocarbon gases in railway or road tanks, from which they are transferred to factory stationary containers.
Small consumers usually use cylinders.
Transport gases in accordance with the rules for the transportation of dangerous goods and the rules for the safe operation of pressure vessels.
First of all, it is worth considering the properties of propane. Its temperature in the cylinder should not exceed 15 degrees Celsius, otherwise there is a risk of fire.
Propane chlorination - an industrial method for producing perchlorethylene
Thermal chlorination of propane (250-350 °C) leads to a difficult-to-separate mixture of mono- and dichloropropanes; when the temperature rises to 400-500 °C, chloropropenes are formed; exhaustive chlorination in excess of chlorine at 550-600 °C is one of the industrial methods for producing perchlorethylene and CC14.
Thermal chlorination of propane in industry is carried out mainly for the purpose of producing 1,3-dichloropropane, from which cyclopropane is obtained. The mechanism of propane chlorination includes the following stages: propane and chlorine are heated separately in liquid form to 400-600°, after which chlorine is introduced into the propane flow at high speed so that the speed of its introduction is higher than the speed of flame propagation. The reaction is carried out in a tubular coil. As with the chlorination of methane, a stepwise supply of chlorine is used in such a way that in the section of the reaction pipe between the previous and subsequent supply of chlorine, the reaction has time to be completely completed. Removal of excess reaction heat is achieved by introducing an inert diluent, such as nitrogen or carbon dioxide, with propane. In some installations, the reaction coil is placed in a bath of molten salts for this purpose. The reaction products are cooled in a coil refrigerator, after which they enter a distillation column for separation. The released hydrocarbons are again sent to the reaction, and the chlorinated hydrocarbons are subjected to repeated rectification to be separated into mono-, di- and polychlorides. Distillation is carried out on several columns.