The word “metal” is commonly associated with heavy weight. But this is not at all true - some metals are so light that they do not even sink in water. And some weigh a hundred times less than foam and still remain durable metal. You may also be interested in an article about the most expensive metals in the world.
Find out everything about the lightest metal
The lightest metals in the world
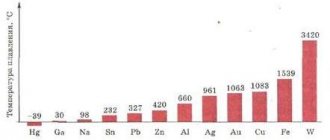
Metals that have low density are called light. This is by no means a rare occurrence. Substances with such characteristics make up approximately 20% of the mass of the earth's crust. They are actively mined and widely used in industry.
The lightest metal is lithium. In addition to the lowest atomic mass, it also has the lowest density, which is two times lower than that of water. After lithium come potassium, sodium, aluminum, rubidium, cesium, strontium, etc. These include titanium, which has the highest strength among metals.
Aluminum is also lightweight and durable. It is the third most common in the earth's crust. Until people learned to obtain it industrially, the metal was more expensive than gold. Nowadays, a kilogram of aluminum can be bought for about $2. It is used both in rocketry and the military industry, and for the manufacture of food foil and kitchen items.
Areas of application
Lithium has unique properties and has many advantages over other metals. People use it in different areas:
- Lithium copper sulfide is an excellent semiconductor intended for the creation of thermoelectric materials.
- The metal, which makes it possible to obtain a dark red flame, is used in the production of pyrotechnics.
- The substance fluoride is widely used in the manufacture of lasers and optics.
- In modern electronics, alkaline batteries with lithium hydroxide are used to obtain maximum power and extend the service life of the products.
- The substance is used as a filler for metal halide lamps.
- Lithium alloys are used in aviation and astronautics.
- In metallurgy, the material is used as an aid in aluminum smelting. The mineral increases the degree of strength and ductility of various alloys.
- Due to its high specific heat capacity, the metal is common in the production of nuclear reactors.
- In the silicate industry, it is necessary for the creation of certain types of glass and for coating porcelain products.
- Lithium hydroxide is used to clean rooms from carbon dioxide.
- Compounds with this substance are used in the textile industry to bleach fabrics.
Various lithium compounds are used in other areas. Since its salts are characterized by healing properties, the substance is widely used in the medical field. Medicines containing this component help in the treatment of mood disorders and dermatological diseases.
Lithium
Lithium is in the first group of the periodic table of elements. It is number 3, after hydrogen and helium, and has the smallest atomic mass of all metals. A simple substance, lithium, under normal conditions has a silvery-white color.
It is the lightest alkali metal with a density of 0.534 g/cm³. Because of this, it floats not only in water, but also in kerosene. For its storage, paraffin, gasoline, mineral oils or petroleum ether are usually used. Lithium is very soft and ductile, easy to cut with a knife. To melt this metal, it must be heated to a temperature of 180.54 °C. It will boil only at 1340 °C.
There are only two stable isotopes of the metal in nature: Lithium-6 and Lithium-7. In addition to them, there are 7 artificial isotopes and 2 nuclear isomers. Lithium is an intermediate product in the reaction of converting hydrogen into helium, thus participating in the process of formation of stellar energy.
History of element discovery
In 1817, the Swedish chemist Johann Arfvedson discovered the substance in the mineral petalite, and then in peroxine spodum and lepidolite mica. And in 1818, Humphry Davy obtained it in the form of a metal during the decomposition of molten lithium hydroxide. When Leopold Gmelin experimented with salts containing this substance, he saw that when the compounds burned, the flame was colored carmine red .
Lithium was first found among hard rocks, so it was given the name lithium, which means “stone” in Greek. The modern name for the metal was given by Berzelius.
Large deposits of lithium have been found in the USA, Bolivia, Chile, Brazil, Congo and China. In Russia, almost 50% of natural mineral reserves can be found in the Murmansk region. The substance was found in ongonites and in highly saline lakes, which were called brines.
Reactions with lithium
Given its alkaline nature, it can be assumed that it is very active. However, metal is the calmest representative of its group. At normal room temperature, lithium reacts weakly with oxygen and many other substances. It shows its “violent temper” after heating, then it reacts with acids, various gases and bases.
Unlike other alkali metals, it reacts mildly with water, forming hydroxide and hydrogen. There is practically no reaction with dry air. But if it is wet, then lithium slowly reacts with its gases, forming nitride, carbonate and hydroxide.
At certain temperatures, the lightest metal is active with ammonia, ethyl alcohol, halogens, hydrogen, carbon, silicon, and sulfur.
Of four percent
The history of the discovery of lithium began with... mathematics. Chemist Arfvedson analyzed a mineral from the Uto mine. The scientist determined that it is an ordinary aluminosilicate, and its content of aluminum, silicon and oxygen is 96%. The persistent chemist wondered what happened to the remaining 4%. By separating the main components and dissolving the residue, he obtained a solution with alkaline properties. It was logical to assume that a new element had been discovered.
The description of the mineral from which the new element was extracted is the words: “ordinary cobblestone.” That’s why they called the new metal lithium (litos in Latin for stone).
Lithium alloys
The properties of lithium enhance certain qualities of metals, which is why it is often used in alloys. Its reaction with oxides, hydrogen, and sulfides is useful. When heated, it forms insoluble compounds with them, which are easy to extract from molten metals, purifying them of these substances.
To give the alloy corrosion resistance and ductility, it is mixed with magnesium and aluminum. Copper alloyed with it becomes denser and less porous, and conducts electricity better. The lightest metal increases the hardness and ductility of lead. At the same time, it increases the melting point of many substances.
Thanks to lithium, the metal becomes durable and resistant to damage. At the same time, it does not weigh them down. That is why alloys based on it are used in space engineering and aviation. Mixtures with cadmium, copper, scandium and magnesium are mainly used.
Physical properties
In the periodic table, lithium (Li) has atomic number 3. The chemical element is located in the second period of the first subgroup. It belongs to the class of alkali metals and is characterized by a light silvery tint. Its properties are determined by the electronic structure of the atom. Lithium belongs to the family of s-elements. Its valency is +1. An atom is characterized by the presence of two shells. The outer layer contains valence electrons involved in the formation of chemical bonds. The nucleus of an atom has a positive charge. It contains 3 protons and 4 neutrons. There are 3 electrons moving in orbits around the nucleus.
Main characteristics of lithium:
- melting point - 180 °C;
- boiling point - 1340 °C;
- density at room temperature - 0.533 g/cm³;
- atomic and molecular mass of the substance - 6.941;
- specific gravity - 0.539;
- Mohs hardness - 0.6.
Lithium is the lightest metal. It can ignite at temperatures above 200 °C. In air it becomes covered with an oxide-nitride film. At room temperature, the substance has a body-centered cubic crystal lattice. A ductile metal is softer than lead and harder than sodium. It can be easily processed by rolling and pressing.
The special physical properties of lithium are due to the small size of its atom. It combines with sodium at a temperature not exceeding 380 °C. Molten potassium, rubidium or cesium do not mix with it.
Finding in nature and meaning
The lightest metal has about 30 minerals of its own, but only 5 of them are used in industry: pentalite, amblygonite, lepidolite, zinnwaldite and spodumene. In addition, it is located in salt lakes. In total, the earth's crust contains 0.005% of this metal.
Large industrial reserves of lithium are found on all continents. It is mined in Brazil, Australia, South Africa, Canada, USA and other countries. After which it is used in electronics, metallurgy, laser materials, nuclear energy and even medicine.
There is a high lithium content in humus, which indicates its participation in the cycle of natural substances. The metal is present in the body of animals, as well as in many plants. Peaches, mushrooms, radishes, potatoes, and carrots are rich in lithium.
In our body it is found in the liver, blood, lungs, bones and other organs. Lack of lithium leads to disturbances in the functioning of the nervous system and brain. It increases the body's resistance to disease and activates the activity of enzymes. It is used to fight Alzheimer's disease, mental disorders, sclerosis, and various addictions.
Place of Birth
In nature, lithium is found in saline solutions (groundwater). Solid sources are often located in pegmatite ores. Minerals: spodumene, lepidolite, elbaite, yadarite.
There are 16 deposits in Russia, but no production is taking place.
World deposits:
- Bolivia;
- Argentina;
- Chile;
- China.
It’s sad: in our country, the demand for lithium-ion batteries is covered by Chinese imports. Or we could produce it ourselves...
Toxicity
Despite lithium's important biological role in our bodies, it can be dangerous. The lightest metal is quite toxic and can cause poisoning. When burned, it provokes irritation and swelling of the mucous membranes. If a piece of whole metal gets on them, the same thing will happen.
Lithium should not be handled without gloves. By interacting with moisture in the air or moisture on the skin, it easily causes burns. You need to be even more careful with molten metal, as its activity increases significantly. When working with it, you need to remember that it is an alkali. You can reduce its effect on the skin with ordinary vinegar.
In the body, lithium increases the stability of the immune system and improves the functioning of the nervous system. But its excess is accompanied by dizziness, drowsiness, and loss of appetite. Metal poisoning leads to decreased libido, muscle weakness, and weight gain. In this case, vision and memory may deteriorate and coma may occur. When working with lithium, you should always wear gloves, a protective suit and goggles.
Production
There are a couple of challenges in lithium brine mining - geography and reliability.
The brine is pumped into “pools” - special ponds, where the content of the element is concentrated by natural evaporation. You need a constantly high temperature (geography) and time - the process takes up to a year. Next, the concentrated brine (1-2% Li) is sent to a chemical plant for processing.
Solid sources are developed using traditional drilling and processing methods.
In the world, four producers control 85% of production (the main ones are Argentina and Chile).
For your information: the largest lithium deposit is in Bolivia; This is the salt marsh of Uyuni. 70% of the world's industrial reserves of the metal are located there.
Lithium. Nirvana
I'm so happy, Because today I found my friends... They are in my head. I'm so ugly, but that's normal, Because you are too... We broke our mirrors. Sunday morning. For me every day. And I'm not afraid, light my candles... I'm in a daze, because I found God...
Yes…
I'm so lonely, but that's okay, I shaved my head... And I'm not sad, And maybe I'm to blame for everything I heard... But I'm not sure... I'm so excited, I can't wait to see you there... I don't care . I'm so excited, But it's not scary, I have good intentions.
Yes..
I love it - I'm not going crazy I miss you, I'm not going crazy I love you, I'm not going crazy I'll kill you, I'm not going crazy...
I'm so happy, Because today I found my friends... They are in my head. I'm so ugly, but that's normal, Because you are too... We broke our mirrors. Sunday morning. For me every day. And I'm not afraid, light my candles... I'm in a daze, because I found God...
Yes…
I love it - I'm not going crazy I miss you, I'm not going crazy, I love you, I'm not going crazy, I'll kill you, I'm not going crazy...
Translated by tester