You can talk about which metal is the softest for quite a long time, if you take into account various parameters. For example, many people know that gold in its pure form is a very soft metal. It is very easy to scratch it with a fingernail or make the thinnest wire from a few grams of this metal.
Thanks to the extraordinary softness of gold, precious items can be made from it in any shape, which is what jewelers use. However, gold is not the softest metal. But gallium can be called this with confidence.
How soft is gold, and how is this property of the metal used?
This metal is quite dispersed in nature. Gold is found in sea water, in the human body, and can be found in almost any fragment of granite. And this despite the fact that gold is only millionths of a percent in the earth’s crust.
In its pure form, the color of this metal is yellow with a hint of red, which distinguishes it from all other metals. Gold is a soft noble metal. It is easily destroyed by the environment.
In its pure form, it is such a soft metal that it is easy to scratch it even with a fingernail. It is known that from one gram of this metal a three-kilometer wire can be drawn, and a gram will also be enough to make the thinnest gold foil, which will be hundreds of times thinner than a human hair, and will bend without breaking.
In terms of mechanical strength and chemical resistance, this precious metal is inferior to almost all platinum group metals, but it is indispensable for electrical contacts. This is why electroplating and gold conductors are widely used in microelectronics. Nuclear research cannot do without gold, where it is used as a target. It is also used in a neutron bomb as a neutron shell.
Source
Areas of application
Noble metals are widely used in a variety of fields. Here are just a few of them
Electrical engineering
Unique physical and technical characteristics in tandem with chemical and biological inertness make it possible to create effective protection of electrical contacts from burning and oxidation. This makes the metal safe and practical for use in electrical engineering.
It is no coincidence that alloys of most precious metals are in widespread demand in the manufacture of high-precision instruments.
Silver salts (chlorides and bromides) are used to create photosensitive elements. Solders made from noble metals are in demand when creating electrical devices, which are subject to increased reliability requirements. The rarest elements are used to create thermocouples and other heating elements.
Jewelry
From time immemorial, precious metals have been in demand in the jewelry industry. They are used to create exclusive chains, earrings, bracelets, rings, pendants, crosses, as well as eyeglass frames, expensive cigarette cases and many other products. Jewelers highly value the color, exquisite shine of metals, as well as their unique properties.
Precious metals do not react with human skin, so they do not lead to skin diseases and allergic reactions. It is allowed to use noble metals as a coating layer for jewelry made from cheap metals. Such jewelry delights its owners for many years and is often inherited from generation to generation.
Chemistry
The resistance of precious metals to acid-base compounds, as well as catalytic parameters, make their use in the chemical industry relevant. They are used to create equipment for aggressive compounds. Many of these metals are used as catalysts in the production of gasoline.
Automotive industry
Catalysts are also used to create exhaust devices. That is why noble metals are in demand in the manufacture of auto parts. They allow you to quickly and reliably neutralize toxic chemical compounds. Most often, palladium and rhodium are used for these purposes.
Medicine
Biological and chemical inertness make it possible to use noble metals in the production of surgical instruments and all kinds of parts for medical equipment. Many metals are in demand in prosthetics and dentistry. A number of compounds have become widespread in the manufacture of medicines as an integral component.
Space Science
Precious alloys are relevant in the construction of aircraft and spacecraft, since only they can ensure maximum reliability and safety of these systems. Only a noble metal can cope with the loads that a space station may experience in orbit.
Glass industry
Precious metals have also found their use in glass production. Very often they are used to make glass melting tanks.
Banking sector
It is also impossible not to mention the role of precious metals as a monetary exchange measure. In ancient times, gold and silver were used to make coins, although nowadays silver has already lost its function in this circulation. And yet investment bars are still cast from gold and platinum to this day.
This allows everyone to invest their free funds with high profits. As practice shows, traditional currency depreciates over time, while gold bars invariably remain in value.
Nowadays, anyone can invest their savings in precious metals of the highest standard.
Many banking and financial organizations even offer depositors to open special metal accounts. These are profitable investments, since in the long term the owners of such bullion can make serious profits. Metal accounts have only one drawback - the lack of a deposit insurance system, which can entail considerable risk if the bank goes bankrupt.
Properties of metals
Iron and its alloys (steel, cast iron), copper, aluminum... The use of these materials marked breakthroughs in scientific and technological progress at different stages of the development of civilization. Each of these metals has characteristics that give it unique practical value. Common features for them are high thermal and electrical conductivity, plasticity - the ability to maintain integrity during deformation, metallic luster.
A damask blade that cuts through iron armor, and the softest metal, on which traces remain from the slightest impact, have a similar internal structure. It is based on a crystal lattice, at the nodes of which there are atoms with a positive and neutral charge, between which there is an “electron gas” - particles that have left the outer shells of the atoms due to a weakening of the bond with the nucleus. A special metallic bond between positive ions located at the nodes of the crystal lattice is carried out due to the attractive forces arising in the “electron gas”. The hardness, density, and melting point of the metal depend on the con.
Industrial Applications
Noble metals are widely used in industry. High-temperature thermocouples are made from platinum-rhodium alloy (90%Pt - 10Rh), which can operate up to 1700°C. The platinum-based alloy is used to make oxygen probe tips for monitoring the protective atmosphere in heat treatment furnaces. Alloys based on palladium, iridium, and silver are used in contacts of critical electronic products. If high wear resistance and hardness are required from a part, then an alloy of osmium and iridium is used.
Purpose of soft metal
From a biological point of view, this metal does not have a special role. But ever since it was first discovered (in 1875), gallium has been used in microelectronics. It is also widely used in pharmaceutical production. Nowadays, gallium arsenide is used to create microwave circuits and infrared applications.
In addition to the fact that this metal is recognized as the softest, it is also expensive. For example, in 2005, for 1000 kilograms of this metal, buyers had to shell out a little more than one million dollars.
Where can I buy or sell?
The main lots on the market remain platinum and gold. If you represent a company or have an individual entrepreneur, it is more profitable to buy metals from officially registered brokerage companies that work with leading manufacturing plants.
Which precious metal is the most expensive?
If we are talking about the most popular and popular metals, then the leading place in the price ranking is occupied by palladium, platinum and gold.
However, Californian is rightfully considered the most expensive metal on earth.
It is mined during the operation of powerful nuclear reactors. The price for 1 gram of Californian is 6.5 million dollars. Next on the list of expensive metals is rhodium. It is estimated at 225 thousand dollars per gram.
Sources
- https://zhazhdazolota.ru/interesnoe/blagorodnye-metally
- https://BusinessMan.ru/new-dragocennye-metally-opisanie-vidy-perechen-i-xarakteristiki-monety-iz-dragocennyx-metallov.html
- https://VseoMetallah.ru/raznoe/blagorodnye-metally
- https://stone-stream.ru/metally/blagorodnye.html
- https://vplate.ru/metally-i-splavy/vse-o-blagorodnyh/
- https://HeatTreatment.ru/blagorodnye-metally-svojstva-i-primenenie.html
Criteria for evaluation
The answer to the question of which metal is the softest will always be a subject of discussion unless the evaluation criteria are agreed upon and the very concept of softness is defined. Opinions about this material characteristic will vary among specialists in different industries. A metallurgist may understand softness as increased malleability, a tendency to accept deformations from abrasive materials, etc.
It is important for materials scientists to be able to objectively compare different characteristics of substances. Softness should also have generally accepted evaluation criteria. The softest metal in the world must have generally recognized indicators proving its “record” characteristics. There are several techniques whose purpose is to measure the softness of various materials.
Story
The concept of “rare metals” has come into use since the mid-1920s. At that time, this was the name given to elements without their own deposits, scattered in an array of other ores.
Sometimes the terms “rare metal” and “rare element” are equated. This is mistake:
- Rare elements are a broader concept.
- It includes metals, non-metals, inert gases.
- Of the six dozen positions on the list of rare elements, metals account for 50.
The second name of this group is less common (usual) metals.
Measurement methods
Most certified methods for measuring hardness are based on the contact effect on the test material from a harder body called an indenter, measured using precision instruments. Depending on the type of indenter and the measurement methods, there are several main methods:
- Brinell method. The diameter of the imprint left by a metal ball when pressed into the surface of the test substance is determined.
- Rockwell method. The depth of indentation into the surface of the ball or diamond cone is measured.
— Vickers method. The area of the imprint left by the diamond tetrahedral pyramid is determined.
— Shore hardness. There are scales for very hard and very soft materials - the depth of immersion of a special needle or the height of rebound from the surface of a special striker is measured.
Product marking
In the production of technical products for various purposes and jewelry, precious metals are used exclusively in the form of alloys with other metals.
Precious metal alloys get their name from their main component (gold alloys, platinum alloys, etc.). The main materials used for jewelry are gold, silver, platinum and palladium.
Other platinoids (rhodium, osmium, iridium and ruthenium) are added to precious metal alloys in order to adjust the physical and technical properties of the product being manufactured - color changes, ductility and hardness, heat resistance and melting point.
To identify products containing precious metals, markings are used (from German markieren - to put a mark, a symbolic mark) with marking information applied in the following ways:
- using a mark (branding) directly on the surface of the product - for jewelry;
- entries in product passports, forms, labels, operating manuals, reference books and catalogs.
Purpose and methods of branding
According to the “Regulations on hallmarking and hallmarking of products made of precious metals...” (Decree of the President of the Russian Federation dated October 2, 1992 No. 1152), all jewelry and household products made from precious metals for sale must be branded with the state hallmark (clause 2 ).
The mark is applied only when the jewelry is made in accordance with all official canons and meets state quality standards.
The absence of a mark clearly indicates that it has not passed the inspection of the state inspection service, or was simply not presented to it and is being sold in circumvention of legal regulations.
Another marking on the product is the name tag, which is an imprint designating the manufacturer who manufactured the product.
Selling jewelry made of precious metals without a name stamp and inspection by the assay service is illegal.
At its core, hallmarking is an assay-technological operation of applying an hallmark to products made of precious metals. The method of its application is determined by the degree of complexity of the shape and purpose of the product on which the marking must be applied.
Currently, three branding methods are used:
- A mechanical or impact method of applying a mark, based on mechanical action on the surface of the jewelry (hitting a tool with a mark on the tip with a hammer after applying the mark to the surface of the product). Until now, about 80% of all products made from precious metals are branded in this way. Only the hammer has been replaced by automated stamping machines.
- Electroerosive or electro-spark method, which is essentially the burning of a mark with an electric spark. This branding technique is convenient for working with fragile hollow products. About 10-20% of all jewelry products are branded using the electrospark method.
- Laser method, in which the outline of the mark is applied with a laser. This mark is small in size, but has clear outlines. It is considered the only way to brand convex and concave hollow products.
How to read the mark?
The mark consists of two marks, which are either applied separately or presented together on one print:
- images of the sample mark;
- identification design - a five-pointed star with a hammer and sickle (for products produced before 1994) or a female head in a kokoshnik with a profile turned to the right for products of a later release, as shown in the table below.
The presence of an identification mark indicates that it has been assayed and stamped by the Assay Supervision Inspectorate.
The shape of the mark depends on the type of noble alloy. Below are sketches of hallmarks:
What makes precious metals strong?
Only thanks to the ligature do gold and silver become suitable for making jewelry, and in the case of silver, cutlery. What is this, a ligature? In simple terms - impurities of other metals that add strength.
The gold ligature contains 41.5% of 100% of the total metal in the product. From this the most common test was born, i.e. the remaining 58.5% is converted into the 585 hallmark. Of course, there is also the 375 and 750 hallmarks. The gold of such hallmarks is, respectively, stronger and less durable than the 585 hallmark.
In the ligature of the most common gold standard, i.e. 585, includes: copper, silver, nickel and palladium. Depending on the shade of the product, you can guess which additional metal is more. In sample 375, only copper and silver are present, but in sample 750, on the contrary, platinum is also added to the list of metals of sample 585.
If we consider silver in the same way, then the alloys in it are 7.5%, i.e. The product contains 92.5% pure silver, which corresponds to 925 purity. Like gold, white metal also has different grades. But they are completely unsuitable for jewelry.
The ligature for jewelry silver is copper. This is what makes jewelry more durable. Due to the low content of this metal, the red color does not appear in any way in the alloy with silver.
Soft metals list - Metalist's Handbook
Non-ferrous is a group of different metals and their alloys.
Let's take a closer look at what non-ferrous metal scrap is.
There are two groups of metals:
Iron and its alloys are called black
The rest are non-ferrous or non-ferrous.
Their list is diverse:
- aluminum;
- copper;
- nickel;
- manganese;
- titanium;
- zirconium, etc.
are in demand today both in production and in scientific activities . Their areas of application are varied.
Scrap metal collection points are happy to buy non-ferrous metal scrap at competitive prices, and in order to avoid getting into trouble when handing it over, you need to be familiar with the types and know the standard classification of non-ferrous metals.
Classification of non-ferrous metals according to GOST
The current GOST 1639-2009 clearly indicates what belongs to non-ferrous metal scrap.
The classification of scrap is divided into four main sections that characterize it:
- Name;
- physical parameters;
- chemical composition;
- quality.
GOST metals and their alloys.
The section displays 13 types that are accepted in organizations for receiving recyclable materials.
Below is a table in which you can see a list of non-ferrous metals in one list and the number of individual types of scrap:
Pure metal can rarely be found , since most scrap is made up of alloys.
Upon acceptance, belonging to one or another type is assessed by the element that is greater in percentage terms in recyclable materials.
This ratio can be determined using special equipment.
Non-ferrous metal scrap is divided into types according to the following criteria :
- origin;
- chemical composition;
- physical state.
The origin of the scrap may be as follows:
- industrial waste;
- marriage;
- substandard;
- scrap of finished products.
The chemical composition of non-ferrous metal scrap, which is determined in the laboratory, shows which metal or alloy it belongs to.
The most valuable recyclable materials are unalloyed metals with a low content of impurities. Physical parameters are just as important when passing as chemical ones.
According to these characteristics, scrap is divided into the following classes :
- A – directly refers to scrap and lump waste;
- B – includes shavings, tangled wire and small pieces;
- B - powdered waste (mainly found only in rare metals: tungsten, cobalt, molybdenum and titanium);
- G - other recyclables.
Safety
All non-ferrous scrap must be checked for:
- presence of radiation and harmful chemical contamination;
- explosion hazard.
When transporting scrap metal, it must be accompanied by documentation on radiation and explosion safety.
The concentration of harmful substances must not exceed the values specified in GOST 12.1.005.
The Russian Ministry of Natural Resources has identified five classes of chemical, radiation and explosion hazards of non-ferrous metal scrap:
- Hazardous waste with great harm to the ecosystem. These include mercury, polonium and plutonium.
- Highly hazardous waste, the consequences of which take nature thirty years to remove. These are alloys of lead, cobalt and molybdenum.
- Moderate danger , in which it takes ten years to restore the ecology. This is scrap mixed with copper, nickel, iron, zinc, aluminum and silver.
- Low hazardous waste, removal of the consequences takes three years. This includes scrap bronze.
- Low danger , such scrap does not harm the environment. This is the most common class among colored scrap.
Due to the expected harm to humans and nature, all operations with non-ferrous scrap require a license from the points accepting secondary non-ferrous metals. Checking for all types of hazards is carried out according to the following scheme:
Quality
GOST specifies quality parameters that determine the grade of scrap.
characteristics are of great importance here :
- scrap size;
- origin of scrap;
- uniformity;
- amount of blockage;
- chemical composition;
- physical state;
- dimensions and volume.
Quality is determined on a representative sample.
According to GOST, all transported scrap must be marked with the following indication:
- names;
- GOST designations;
- designations of the type of recyclable materials;
- alloy grades.
Marking of non-ferrous metals and alloys must be firmly attached to the cargo during transportation and storage.
To determine the grade of metal, you need to look at the stamp book , a special document with all the markings of the metal or alloy you are interested in.
Kinds
The large number of non-ferrous metals and various characteristics required their classification into separate types.
industrial systematization is in use , reflecting the historically established components of the metallurgical industry and the science of the same name.
The name itself does not fully reflect the essence of non-ferrous metal.
Only gold and copper are colored, while the rest are the usual grey-black shades.
Science usually distinguishes the following types of non-ferrous metals and alloys:
- lungs;
- heavy;
- noble;
- refractory;
- scattered;
- rare earth;
- radioactive.
industry in Russia today is on the rise and includes:
- metal mining;
- ore beneficiation;
- metal smelting
The main non-ferrous metals include:
Aluminum is an excellent electrical conductor. It is flexible, which is both its advantage and disadvantage.
To give it strength add :
- manganese;
- copper;
- magnesium, etc.
Such alloys are used for the production of :
- airplanes;
- sea and river ships;
- space shuttles;
- in construction;
- in the food industry.
Aluminum and its alloys are the cheapest type of non-ferrous metal scrap.
You can find it in a variety of household items, including:
- siding;
- gutters;
- roofing
Copper is a commonly found non-ferrous metal.
It also has good characteristics:
- plastic;
- good electrical conductor;
- good heat conductor.
It is in great demand in alloys and is used in various economic sectors.
Its alloy with zinc and tin is known - brass.
It can be found in:
- cars;
- hours;
- expensive jewelry.
find copper for scrap metal in:
- power cables;
- water pipes;
- household products.
Copper is highly valued at recycling centers.
Rare
Rare earth metals are used to improve the qualities of other metals and became widely used with the development of industrial production in the 20th century.
These are the following metals:
- scandium;
- yttrium;
- lanthanides.
The name itself suggests that there is very little of these non-ferrous metals in the earth's crust. Also, previously, refractory oxides that form rare non-ferrous metals were called “earths” . They are extracted from oxides.
Today, rare earth metals can be found in all digital devices:
- smartphones;
- players;
- computers;
- in hybrid engines;
- in other electronics.
Alloys made from them have high characteristics , for example:
- anti-corrosion;
- strength;
- heat resistant.
Let's consider heavy non-ferrous metals, collecting them in several lists.
The heaviest non-ferrous metals on Earth:
Rarely found in soil , it is generally the most expensive non-ferrous metal.
Also included in this group are:
- copper;
- lead;
- zinc;
- tin;
- nickel.
All of them have a high density and, accordingly, a lot of weight, which is why they get the name – heavy.
Lead is widely known and used in many industries , contained in:
It is made from:
Lead is also used to create protective aprons from radiation .
Has the following characteristics:
- low thermal conductivity;
- plastic;
- toxicity.
Therefore, lead must be used carefully, following all safety regulations.
Tin used to be called an alloy of lead and silver.
Today, tin is used in the metallurgical industry and the production of various alloys, which include:
- bearings;
- packaging foil;
- bronze;
- food tin;
- wires
Nickel is a heavy non-ferrous metal with high heat-resistant and anti-corrosion characteristics. Nickel is used in alloys. In stainless steel it is the main component.
Made from nickel :
- coins;
- armor;
- chemical equipment;
- wire;
- foil;
- a thread;
- powder;
- alkaline batteries.
in demand in:
- shipbuilding;
- electrical engineering.
Lungs
The definition of “light non-ferrous metals” includes metals with low density.
List of the most popular light non-ferrous metals:
- aluminum;
- tin;
- magnesium;
- titanium;
- beryllium;
- lithium.
The lightest non-ferrous metal is lithium. It is widely used in various alloys.
is used in:
- chemical industry;
- metallurgical industry;
- military-industrial complex;
- thermonuclear energy.
Lithium is also used in the manufacture of:
- optics;
- alkaline batteries;
- ceramic products.
The ductility of magnesium is not as good as that of copper and aluminum, which affects the welding properties of this metal. But it can be easily cut with a special tool. At the same time, the mechanical properties leave much to be desired. This it to be widely introduced into industrial production .
Popular types at collection points
The most popular non-ferrous metals at collection points:
If you want to find out what is more profitable to rent out, then read this article.
Conclusion
Historically, non-ferrous metals are divided into types:
- lungs;
- heavy;
- noble;
- rare earths, etc.
This classification is accepted in technology; today it meets almost all the requirements of the industrial industry.
This makes it easier:
- the task of producing new alloys;
- delivery of secondary raw materials from non-ferrous metal at collection points.
Each operation for the acceptance and delivery of non-ferrous metal must be confirmed by an act that states:
- compound;
- quality;
- volume;
- cost per kilogram and for all scrap.
When handing over scrap, make sure that is honest . Many small points deliberately underestimate the grade of scrap or weigh it using incorrectly working scales.
Mohs hardness scale
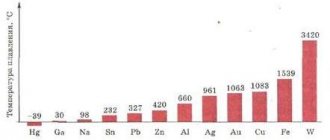
This scale for determining the relative hardness of minerals and metals was proposed at the beginning of the 19th century by the German Friedrich Mohs. It is based on the scratching method, where a harder sample leaves a mark on a softer one, and is very convenient for finding out which metal is the softest. In relation to 10 reference minerals, which are assigned a conditional hardness index, the place on the scale and a digital index are determined for the test substance. The softest reference mineral is talc. It has a Mohs hardness of 1, and the hardest, diamond, is 10.
Investing in precious metals
Is it profitable to invest hard currency in a bank? Precious metals are the most stable currency, which, if successfully invested, can increase the owner’s profit, as well as protect him from market fluctuations. All over the world, banks practice opening impersonal metal accounts, which can be held as deposits or participate in exchange transactions. Banking transactions involve: gold, platinum, palladium and silver.
the hardest metal on planet earth?
Many lovers of interesting facts are interested in the question, which metal is the hardest? And it won’t be easy to answer this question offhand. Of course, any chemistry teacher will easily say correctly, without even thinking. But among ordinary citizens who last studied chemistry at school, not many will be able to give the answer correctly and quickly. This is due to the fact that since childhood everyone has been accustomed to making various toys from wire and has well remembered that copper and aluminum are soft and bend well, but steel, on the contrary, is not so easy to give the desired shape. A person deals with the three named metals most often, so he doesn’t even consider the other candidates. But steel is certainly not the hardest metal in the world. To be fair, it is worth noting that this is not a metal at all in the chemical sense, but a compound of iron and carbon. The hardest metal is titanium. Pure titanium was first obtained in 1925. This discovery created a sensation in scientific circles. Industrialists immediately drew attention to the new material and appreciated the benefits of its use. According to the official version, the hardest metal on Earth got its name in honor of the indestructible Titans, who, according to ancient Greek mythology, were the founders of the world. I, too, thought - tungsten https://promplace.ru/vidy-metallov-i-klassifikaciya-staty/samyi-tverdyi-metall-1494.htm But no. And a dozen metals whose hardness is 5 units and above look like this: iridium – 5; ruthenium – 5; tantalum – 5; technetium – 5; chromium – 5; beryllium – 5.5; osmium – 5.5; rhenium – 5.5; tungsten – 6; uranium – 6 https://thedifference.ru/samyj-tverdyj-metall/ https://ru.wikipedia.org/wiki/Metals
The hardest of the purest metals on the planet is chromium.
1. Not hard. and hard. 2. Not planet earth, but planet Earth. Metal? This is tungsten. But there are alloys of other metals that are much harder.
polyubas is not a metal but an alloy because even steel is an alloy of metals. And why does it have to be metal? If on the Rockfeld scale hardness is measured with a diamond cone, then diamond is accepted as the standard.
WHAT IS METAL ON PLANET EARTH? Are there other metals on Jupiter, or what?
Analysis
The noble metal analyzer aims to answer two main questions:
- what kind of raw material is in front of us: pure precious metal or an alloy with an insignificant content of a noble element;
- what is the percentage of precious metal in the alloy mass presented for analysis.
The first sample is qualitative, the second gives a quantitative result. They are performed in strict sequence, one after another. After conducting a qualitative test, establishing that the alloy actually contains a precious metal, you can proceed to determining its quantity. If, when examining the analyzed sample by reacting with assay acid, nothing remains, then it is a base metal.
The results established during the examination were reflected in the samples. This is a numerical marking; it shows the percentage of precious metal in the presented alloy.
However, it should be borne in mind that the test in Russia is not applied to all alloys, but only to those in which the concentration of the noble element is more than 30%.
The softest are alkali metals
From the Mohs mineralogical scale it is clear that the softest substances are those belonging to the alkali metals. Even mercury, familiar to many from the liquid from a thermometer, has a hardness index of 1.5. Softer than it are several substances that have similar physical, mechanical and chemical properties: lithium (0.6 on the Mohs scale), sodium (0.5), potassium (0.4), rubidium (0.3). The softest metal is cesium, which has a Mohs hardness rating of 0.2.
The physical and chemical properties of alkali metals are determined by their electronic configuration. It differs only slightly from the structure of inert gases. An electron located at the outer energy level has mobility, which determines high chemical activity. The softest metals are particularly volatile and difficult to mine and preserve unchanged. They are characterized by violent chemical interactions with air, water, and oxygen.
All about metals - types of metals and alloys
Metals
and their alloys are one of the main structural materials of modern civilization. This is determined primarily by their high strength, uniformity and impermeability to liquids and gases. In addition, by changing the alloy formulation, it is possible to change their properties within a very wide range.
Almost all metals of industrial importance are used in the form of alloys. All smelted iron is almost entirely used for the production of ordinary and alloy steels, as well as cast iron. The fact is that by alloying with certain components, the mechanical, electrical, magnetic, and thermal properties of many metals can be significantly improved.
People learned about the existence of metals at the dawn of civilization.
They discovered that some stones, which were later called ore, when heated turned into a shiny substance - a metal that was a liquid at high temperatures and a solid at room temperature.
For the practical use of metals and the manufacture of metal products (weapons, agricultural implements), people learned to process metal. The first metal processing technologies emerged.
Metallurgy is a field of science and technology and a branch of industry that covers the processes of obtaining metals from ores or other materials, changing the chemical composition, structure and properties of metal alloys, and giving the metal a certain shape.
Metallurgy is one of the most dynamically developing sectors of the world economy. The development of metallurgy production capacities is noticeably ahead of the growth dynamics of the global industry. Competition in the industry is high, and supplies of imported products are increasing. Thus, according to IISI, world steel production in 2006
amounted to 1.219 billion tons, which is 7.93% higher than the previous year.
The section contains background information about the main industrial metals: occurrence in nature, physical and chemical properties, production and industrial use of metals, as well as the biological effects of metals and their compounds on humans. You can select the metal you are interested in by clicking on the link in the left menu of the page.
Currently, there is a basic classification of types of metals, defining three main groups: ferrous, non-ferrous and noble metals. The latter are sometimes included in the group of non-ferrous metals. Ferrous and non-ferrous metals are used in almost all areas of industrial production; noble metals also serve as a means of preserving and accumulating value.
Black metals
Ferrous metals are iron and its alloys. This includes all types of cast iron and steel. Ferrous metals also include manganese and vanadium.
The process of producing ferrous metals is called ferrous metallurgy. Casting iron and steel has traditionally been considered one of the constituent parts of the industrial power of any large country.
The production of refractory metals and ferroalloys also belongs to the field of ferrous metallurgy.
Non-ferrous metals
Another type of metals
, non-ferrous metals, includes most of the metallic elements of the periodic table, with the exception of ferrous metals and heavy transuranium elements. This group includes the familiar copper and tin, aluminum and titanium, tantalum and zirconium, as well as rare earth elements. Non-ferrous metals are divided into two large groups: heavy and light non-ferrous metals.
Heavy ones are primarily copper, nickel, lead, zinc, and light ones are aluminum, magnesium, titanium. Smelting hot non-ferrous metals requires a huge amount of energy, which is why aluminum is sometimes called the metal equivalent of electrical energy. The Russian Federation has almost the entire range of non-ferrous metals and is the leading world power in the field of non-ferrous metallurgy.
Noble metals
Among all types of metals, noble metals have the property that they are practically not susceptible to oxidation. The group of noble metals includes gold, silver, platinum, palladium and other platinum group metals. All noble metals are characterized by increased chemical resistance and brilliance.
The main areas of application of this type of metal are the jewelry industry, electronics, medicine, the production of protective surfaces and catalysts.
Due to their high cost and resistance to the external environment, metals in this group (mainly gold) are used as a store of value; states accumulate gold as a conservative part of their gold and foreign exchange reserves.
Assortment of metal products “Ruler - S”
The hardest metal is chromium and titanium.
Chromium is an element of the secondary subgroup of the sixth group of the fourth period of the periodic system of chemical elements of D.I. Mendeleev, with atomic number 24. It is designated by the symbol Cr (Latin: Chromium). The simple substance chromium (CAS number: 7440-47-3) is a hard metal of a bluish-white color.
Chromium occurs in nature mainly in the form of chromium iron ore Fe(CrO2)2 (iron chromite). Ferrochrome is obtained from it by reduction in electric furnaces with coke (carbon): FeO Cr2O3 + 4C → Fe + 2Cr + 4CO↑
Chromium is a fairly common element; its content in the earth's crust is approximately 0.02% (22nd place). Ferrochrome is used for the production of alloy steels. To obtain pure chromium, the reaction is carried out as follows: 1) iron chromite is fused with sodium carbonate (soda ash) in air: 4Fe(CrO2)2 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2↑ 2) sodium chromate is dissolved and separated from iron oxide; 3) convert the chromate to dichromate, acidifying the solution and crystallizing the dichromate; 4) pure chromium oxide is obtained by reduction of dichromate with coal: Na2Cr2O7 + 2C → Cr2O3 + Na2CO3 + CO↑ 5) metallic chromium is obtained using aluminothermy: Cr2O3+ 2Al → Al2O3 + 2Cr + 130 kcal 6) electrolytic chromium is obtained from a solution of chromic anhydride using electrolysis in water containing sulfuric acid. In this case, mainly 3 processes take place at the cathodes: reduction of hexavalent chromium to trivalent chromium with its transition into solution; discharge of hydrogen ions with the release of hydrogen gas; discharge of ions containing hexavalent chromium with precipitation of metallic chromium; Cr2O72− + 14Н+ + 12е− = 2Cr + 7H2O
Production of chromium The raw material for the industrial production of chromium is chromium iron ore. Its chemical processing leads to Cr2O3. Reduction of Cr2O3 with aluminum or silicon produces chromium metal of low purity: Cr2O3+Al=Al2O3+2Cr 2Cr2O3+3Si=3SiO2+4Cr Purer metal is obtained by electrolysis of concentrated solutions of chromium compounds.
Titanium - (Latin Titanium; denoted by the symbol Ti) - an element of the secondary subgroup of the fourth group, the fourth period of the periodic table of chemical elements of D.I. Mendeleev, with atomic number 22. The simple substance titanium (CAS number: 7440-32-6) - light metal of silvery-white color. Exists in two crystal modifications: α-Ti with a hexagonal close-packed lattice, β-Ti with cubic body-centered packing, α↔β transition temperature 883 °C
The softest metals are potassium, rubidium, cesium .
Potassium is an element of the main subgroup of the first group, the fourth period of the periodic system of chemical elements of Mendeleev D.I., with atomic number 19. Denoted by the symbol K (Latin Kalium). The simple substance potassium (CAS number: 7440-09-7) is a soft alkali metal with a silvery-white color. In nature, potassium is found only in combination with other elements, for example, in sea water, as well as in many minerals. It oxidizes very quickly in air and very easily enters into chemical reactions, especially with water, forming an alkali. In many respects, the chemical properties of potassium are very similar to sodium, but in terms of biological function and use by the cells of living organisms, they are still different.
Application of noble metals in the automotive industry
If you open the car’s operating manual, then in almost every one of them you can find information about which components and in what quantities precious metals are used.
The main metals used in cars are silver, gold, palladium and platinum. Thus, according to the operating manual for LADA 110 cars from 2007, cars of the tenth family use 0.103 grams of palladium, less than 0.1 grams of gold and more than 3 grams of silver. These metals are mainly used in contacts and conductors in the production of relays, breakers, sensors or control units. GAZ cars, for example model 2217, do not use palladium, but about 0.1 grams of platinum and more than 5 grams of silver are used. Even more precious metals are used in the production of KAMAZ trucks.
Item #55
The name “cesium” comes from the Latin caesius - “sky blue”: in the spectrum emitted by a highly heated substance, two bright blue stripes are visible in the infrared range. In its pure form, it reflects light well, looks like light gold and has a silvery-yellow color. Cesium is the softest metal in the world, the Brinell hardness index is 0.15 Mn/m2 (0.015 kgf/cm2). Melting point: +28.5°C, therefore, under normal conditions, at room temperature, cesium is in a semi-liquid state.
It is a rare, expensive and extremely reactive metal. In electronics, radio engineering and the high-tech chemical industry, cesium and alloys based on it are increasingly used and the need for it is constantly growing. Its chemical activity and ability to form compounds with the highest electrical conductivity are in demand. Cesium is an important component in the production of special optical devices, lamps with unique properties and other high-tech products. At the same time, softness is not its most sought-after quality.
Gallium: characteristics
This element is a fairly ductile metal, which is silver in color and has a bluish tint. The softest metal in the world is located on the periodic table at number 31. In nature, this metal is not found in its pure form, but is extracted from zinc ore or bauxite, which contain gallium in huge quantities.
However, gallium can hardly be considered the softest if it is exposed to low temperatures. In this case it is very hard. But as soon as the air temperature rises to plus 29.8 degrees, gallium begins to melt. To melt such metal, you can only put it in your hand.
And if a spoon made of gallium ends up in hot tea, the melting process will go even faster. At a temperature of plus 500 degrees, this metal becomes so “aggressive” that it is capable of corroding many metals (with the exception of tungsten). For example, if a drop of gallium heated to such a temperature ends up on an aluminum can, then after about thirty minutes the structure of the can weakens and it crumbles, like thin ice from mechanical stress.
It is even possible to observe how gallium resembles a heart that beats - an experiment is performed when a representative of the softest elements begins to perform active movements and even resembles a completely unknown form of life. And it happens like this - molten gallium in the form of one drop comes into contact with the tip of the nail. In this case, the drop first continues to melt, and then, when the contact ends, it gathers again. The result is quite a spectacular spectacle that you want to watch and watch.
Advantages and disadvantages
There are many advantages, but no less disadvantages.
| Advantages | Flaws |
| Easy machining | High density; products turn out heavy |
| Hardness, elasticity, strength - the best properties of alloys | Metal corrosion in the presence of moisture |
| The ability to obtain specified properties of alloys by adding a small amount of impurities | Tendency to electrochemical corrosion |
We recommend: BRASS - an alloy from the legendary Atlantis
Malleability makes it possible to produce decorative products.
Minerals
There is a lot of iron in the form of compounds and minerals on Earth. It is the second most common metal.
| Iron containing minerals | Name, Fe content (in %%) |
| Hematite (red iron ore) | Up to 70 |
| Magnetite (magnetic iron ore) | 72 |
| Siderite | 35 |
| Marcasite | More than 46 |
| Mispickel | 34 |
| Goethite | 62,9 |
Iron ores are divided into 11 industrial types.
Iron ore
We are proud: Russia has the most iron ores of all countries.
What is considered "less common" material
Rare metals include an element that meets at least one criterion:
- Low occurrence in the lithosphere, scattered without primary deposits.
- Complex technology for extracting from ore and obtaining a pure substance.
- Novelty, lack of mastery of the material for practical use.
The last condition is the most mobile. The development of technology, the emergence of new areas of use, and the scaling of production transform the element into familiar ones.
Receiving technology
Rare metals are isolated from metallurgical waste.
The process is standard:
- Enrichment of raw materials.
- Isolation, separation of components.
- Cleaning.
- Recovery.
Metallothermy, electrolysis, and smelting are used.
The refractory group is affected by powder metallurgy methods.
Rare earth metals are “ separated ” by extraction. Catalysts are ion exchange processes and organic solvents.