Aluminum is a metal that is widely used in industry and everyday life.
It is used to produce not only aircraft and ship parts, but also dishes and other utensils. Therefore, there is often a need to independently manufacture aluminum parts that have failed.
The ability of aluminum to melt at relatively low temperatures makes it possible to produce cast products from it in artisanal conditions. In order to independently produce cast aluminum products, you need to know the behavior of this metal at high temperatures and its physical and chemical properties.
Characteristics of aluminum
The melting point of aluminum depends on the purity of the metal and is approximately 660 °C. Its boiling point is 2500 °C.
Aluminum is distinguished by its lightness and ductility, so it bends well and can be processed by stamping.
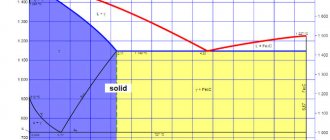
This metal is an excellent conductor of heat and actively enters into a chemical reaction at high temperatures with atmospheric oxygen, forming an oxide film on the surface. It protects aluminum from further oxidation, but when scrap melts, it significantly affects the composition of the alloy. During the metal smelting process, the structure of aluminum changes.
When it cools sharply, internal stresses and shrinkage of the resulting alloy may occur. This must be taken into account when working with aluminum at home.
Technologies for home aluminum casting and necessary equipment
The principle of casting aluminum at home should be based on the technology for its production in production, adjusted for conditions that can be used at home.
Aluminum products are produced by casting in several ways. In domestic conditions, the most common and convenient method is the technology of casting molten aluminum into specially made molds.
Therefore, to carry out the process, two things must be ensured:
- build a furnace for melting aluminum scrap;
- create the desired shape to produce a cast alloy or a separate part.
The casting process must include several stages:
- Preparation of aluminum scrap, including cleaning from dirt, impurities and various fillers, as well as grinding it to a small size.
- Carrying out the smelting process in the planned way. When the metal is completely melted, slag formations must be removed from its surface.
- Filling the prepared mold with liquid aluminum melt. After solidification, the ingot is freed from the molding mass.
Let's consider how to melt aluminum at home, what designs of furnaces for melting metal can be used, as well as options for making molds yourself.
Homemade furnaces and methods for melting aluminum
In order to melt aluminum, you need to heat it to a temperature close to 660 °C. It is impossible to reach such a temperature on an open flame of a fire. Therefore, a closed space is needed, which a homemade stove can provide. It can be heated by burning coal and wood or using natural gas.
You can also use an electric muffle furnace if you have one on the farm.
With a self-made stove, forced ventilation must be provided to maintain the combustion process.
1. The simplest version of a homemade fireplace can be made from old pots.
Its design is as follows:
- As a frame, use a steel container, for example, an old pan, on the side of which you need to make a hole to supply air through a connected metal pipe.
- Air can be forced through the hose using a vacuum cleaner.
- Coal is placed inside the device.
- Then the coal is set on fire and air is supplied to keep the fire from going out.
- A container for melting aluminum is first placed inside an improvised furnace structure and lined with coal on its sides. When it burns, uniform heat distribution is ensured.
- To prevent heat from being lost to the surrounding air, the top of the “pan” stove should be loosely covered with a lid, leaving a small gap for the smoke to escape.
An ideal design would be a firebox with an oval arch made from a masonry mixture used for heat-resistant bricks. You can use a flower pot of the desired size as a frame to create an oval vault.
After the mixture dries, a good firebox is obtained that can withstand several heats.
2. The second version of the furnace involves using the flame of a household gas burner to heat aluminum.
It can only be used for piece products made of aluminum weighing no more than 150 grams. An imitation oven is created by using two containers inserted into each other with a small gap. These can be ordinary cans from canned food.
Read also: DIY garage antenna
The outer jar should be larger. A hole with a diameter of about 4 cm is made in it to ensure the supply of flame to the inner can.
The flame jet should be directed towards the opening of the can. Only the inner container is heated directly, and the outer one serves as a shell that retains heat. The top of the structure must be covered with a simulated lid, leaving a gap for the removal of combustion products.
This design is disposable and can only be used for one melt, since the tin is thin and can quickly burn out.
Aviation
At the present stage of development of subsonic and supersonic aviation, aluminum alloys are the main structural materials in aircraft construction.
Alloys of the 2xxx, 3xxx, 5xxx, 6xxx and 7xxx series are widely used in US aviation. The 2xxx series is recommended for work at high operating temperatures and with increased values of the fracture toughness coefficient. Alloys of the 7xxx series - for operation at lower temperatures of significantly loaded parts and for parts with high resistance to stress corrosion. For lightly loaded components, alloys of the 3xxx, 5xxx and 6xxx series are used. They are also used in hydraulic, oil and fuel systems.
In Russia, in the manufacture of aircraft, high-strength aluminum alloys Al-Zn-Mg-Cu and medium and high-strength Al-Mg-Cu alloys, strengthened by heat treatment, are successfully used. They are a structural material for the skin and internal alloy elements of an aircraft airframe (fuselage, wing, keel, etc.). Alloy 1420, which belongs to the Al-Zn-Mg system, is used in the construction of welded fuselage of passenger aircraft. In the manufacture of seaplanes, the use of weldable corrosion-resistant magnolia alloys (AMg5, AMg6) and Al-Zn-Mg alloys (1915, B92, 1420) is envisaged.
Figure 1 – Civil aircraft
Weldable aluminum alloys have an undeniable advantage when creating space technology objects. High values of specific strength and specific rigidity of the material made it possible to ensure the manufacture of tanks, intertank and nose parts of the rocket with high longitudinal stability. The advantages of aluminum alloys (2219, etc.) include their performance at cryogenic temperatures in contact with liquid oxygen, hydrogen and helium. These alloys undergo so-called cryogenic hardening, i.e. strength and ductility increase in parallel with decreasing temperature.
Alloy 1460 belongs to the Al-Cu-Li system and is more promising for the design and manufacture of tank structures in relation to cryogenic fuels - compressed oxygen, hydrogen or natural gas.
Shipbuilding
Aluminum and its alloys are increasingly used in shipbuilding. Aluminum alloys are used to make ship hulls, deck superstructures, communications and various types of ship equipment.
The main advantage of introducing aluminum and its alloys compared to steel is the reduction in the weight of ships, which can reach 50–60%. As a result, it becomes possible to increase the vessel’s carrying capacity or improve its tactical and technical characteristics (maneuverability, speed, etc.).
The most widely used aluminum alloys for the manufacture of river and sea fleet structures are the magnesium alloys AMgZ, AMg5, AMg61, as well as the alloys AMts and D16. The hull of a high-capacity vessel is made of steel, while the superstructure and other auxiliary equipment are made of aluminum alloys. Fishing longboats are manufactured from AMg5 alloy (sheathing).
Weldable alloys of the 5xxx and 6xxx series are widely used in US shipbuilding. Where high strength (500 MPa) is required, semi-finished products from alloys of the 2xxx and 7xxx series are used.
Receipt
Aluminum is in first place among metals and in third place among all elements in terms of abundance in the earth's crust. Approximately 8% of the mass of the earth's crust is this metal. Aluminum is found in the tissues of animals and plants as a trace element. In nature, it is found bound in the form of rocks and minerals. The rocky shell of the earth, which is at the base of the continents, is formed precisely by aluminosilicates and silicates.
Aluminosilicates are minerals formed as a result of volcanic processes under appropriate high temperature conditions. During the destruction of aluminosilicates of primary origin (feldspars), various secondary rocks with a higher aluminum content (alunites, kaolins, bauxites, nephelines) were formed. Aluminum is included in secondary rocks in the form of hydroxides or hydrosilicates. However, not every aluminum-containing rock can be a raw material for alumina, a product from which aluminum is produced using the electrolysis method.
Aluminum is most often obtained from bauxite. Deposits of this mineral are common in countries of the tropical and subtropical zone. In Russia, nepheline ores are also used, deposits of which are located in the Kemerovo region and on the Kola Peninsula. When extracting aluminum from nephelines, potash, soda ash, cement and fertilizers are also produced along the way.
Bauxite contains 40-60% alumina. It also contains iron oxide, titanium dioxide, and silica. The Bayer process is used to isolate pure alumina. In an autoclave, the ore is heated with caustic soda, cooled, and the “red mud” (solid sediment) is separated from the liquid. Afterwards, aluminum hydroxide is precipitated from the resulting solution and calcined to obtain pure alumina. Alumina must meet high standards for purity and particle size.
Alumina (aluminum oxide) is extracted from the mined and enriched ore. The alumina is then converted into aluminum using electrolysis. The final stage is recovery by the Hall-Heroux process. The process is as follows: during the electrolysis of an alumina solution in molten cryolite, aluminum is released. The cathode is the bottom of the electrolysis bath, and the anode is carbon bars located in cryolite. Molten aluminum is deposited under a solution of cryolite with 3-5% alumina. The process temperature rises to 950°C, which is much higher than the melting point of aluminum itself (660°C). Deep purification of aluminum is carried out by zone melting or distillation through subfluoride.
Railway transport
The harsh operating conditions of railway rolling stock (long service life and ability to withstand shock loads) place special demands on structural materials.
Figure 2 – Freight train
The main characteristics of aluminum and its alloys, revealing the feasibility of their use in railway transport, are high specific strength, low inertial force, and corrosion resistance. The introduction of aluminum alloys in the manufacture of welded containers increases their durability when transporting a number of products from the chemical and petrochemical industries.
Aluminum and its alloys are used in the manufacture of the car body and frame. For the car, weldable medium-strength alloys of the AMg3, AMr5, Amg6 and 1915 grades are recommended. Aluminum alloys are promising alloys for refrigerated cars. Depending on the products of the chemical industry, the brand of welded material for tank boilers is selected.
In the USA, rolling stock is manufactured from weldable alloys of the 6xxx series, 5xxx series and alloy 7005, obtaining optimal strength characteristics and high corrosion resistance of welded elements.
Automobile transport
One of the main requirements for materials used in automobile transport is low weight and fairly high strength indicators. The corrosion resistance and good decorative surface of the material are also taken into account.
Figure 3 – Car
The high specific strength of aluminum alloys increases the carrying capacity and reduces the operating costs of mobile vehicles. The high corrosion resistance of the material extends service life and expands the range of transported goods, including liquids and gases with high aggressive concentrations.
In the manufacture of frame elements and body trim for semi-trailers, vans, refrigerators, livestock carriers, etc. Promising materials are aluminum alloys AD31, 1915 (extruded profiles) and alloys AMg2, AMg5 (sheet).
Aluminum alloys AMts, AMgZ and 1915 are used in the manufacture of individual components of a passenger car (attachment parts, bumpers, cooling radiators, heaters).
In the US automotive industry, aluminum weldable alloys of the 3xxx, 5xxx and 6xxx series are widely used.
Beams and frames of heavy trucks are made from pressed semi-finished alloys 2014 and 6061. Panels and individual elements made from 5052 alloy are used to manufacture the cabin. As a lining material of the truck body, a sheet of alloy 5052, 6061, 2024, 3003 and 5154 is used. The body racks are made of pressed semi -finished alloys of alloys 6061 and 6063. Magnal alloys of the 5XXX series (5052, 5086, 5154 and 5454) are the main material in the manufacture of cargo stations .
Construction
The prospects for using aluminum alloys in building structures are confirmed by technical and economic calculations and many years of world practice in the field of construction of various construction projects.
The introduction of aluminum alloys in construction reduces metal consumption, increases the durability and reliability of structures when operating under extreme conditions (low temperature, earthquake, etc.). Depending on the purpose of building aluminum structures, various grades of alloys are recommended: AD1, AMts, AMg2, AD31, 1915, etc.
Figure 4 – Building with translucent aluminum structures
Experience accumulated in the USA confirms the feasibility of using aluminum alloys in building structures. They use more aluminum than any other industry. In this case, preference is given to the introduction of weldable alloys of the 3xxx, 5xxx and 6xxx series.
What is made of aluminum list
The discovery of aluminum made it possible to create light and strong stairs, build residential and industrial buildings and make durable windows
On this topic
China's daily aluminum production sets record in June
Metallurgical company was attacked by hackers
The chemical element aluminum was first discovered by a Danish physicist in 1825. The discovery of a new substance turned out to be significant. As can be seen from the materials of the site https://www.met-eco.ru/al, subsequently people identified many options and ways to use aluminum in domestic conditions. At first, aluminum contained impurities; the pure element was obtained in 1925 by electrolysis.
Previously, it was assumed that it would be impossible to find pure aluminum under ordinary natural conditions. It was believed that aluminum could be created artificially using human hands. However, aluminum was later found in a free state in the form of thin threads about half a millimeter long. The width of the aluminum finds was only a few micrometers.
Options for using aluminum in everyday life:
- For making kitchen utensils that are light and easy to use;
- Aluminum can be used in the manufacture of household appliances as a durable and lightweight component. Household appliances should not be heavy; lightweight aluminum provides minimal weight to any kitchen equipment;
- Aluminum can be used to produce foil and various containers;
- This element is widely popular in construction activities, where such a simple material is indispensable. Aluminum is used to make metal structures, wires, and profiled sheets;
- Aluminum is often used in the production of automotive devices and the production of machine parts.
Aluminum scrap is always in price due to the existing demand from industrial organizations and individuals. Durable aluminum alloys are often used in housing and communal services.
Recycling of aluminum is useful in the automotive industry. When creating cars, melted non-ferrous scrap is usually used, significantly reducing the price of transport and significantly reducing the weight of the car itself.
In everyday life, you constantly find objects made from this element, for example, cans or foil. The discovery of aluminum made it possible to create light and strong stairs, build residential and industrial buildings, and make durable windows.
Aluminum is a ductile and lightweight white metal coated with a silver matte oxide film. In the periodic system of D.I. Mendeleev, this chemical element is designated as Al (Aluminium) and is located in the main subgroup of group III, third period, under atomic number 13. You can buy aluminum on our website.
Petroleum and chemical industry
The development of new fields and increasing the depth of wells put forward certain requirements for materials used for the manufacture of parts and assemblies of oil and gas field equipment and equipment for processing oil products.
Figure 5 – Oil rig
The high specific strength of aluminum alloys makes it possible to reduce the weight of drilling equipment, facilitate their transportability and ensure the passage of deep wells.
Corrosion-resistant aluminum alloys make it possible to increase the operational reliability of drilling, tubing and oil and gas pipelines. Increased resistance to corrosion cracking makes it possible to use aluminum alloys in the manufacture of tanks for storing oil and its products.
The main structural material in the manufacture of drill pipes from aluminum alloys is D16 alloy.
Aluminum alloys AMg2, AMr3, AMg5 and AMg6 have shown high resistance to crude oil and some gasolines. Of the listed magnalium alloys, the most technologically advanced alloy for the manufacture of apparatus is the AMg2 alloy, especially in the manufacture of capacitors and refrigerators at oil refineries.
In the USA, equipment for the oil industry is made from aluminum alloys of the 3xxx, 5xxx and 6xxx series. In the design of drilling equipment, pipes made of alloy 6063 are used. Offshore platforms are assembled from pipes 6061, 6063, as well as from high-strength alloys of grades 2014 and 7075. Tanks, columns, condensers, etc. are made from aluminum ADOO, ADO and AD1. for the production of acetic acid, sulfonation of fatty alcohols, potassium chlorate, sodium and ammonium nitrate, hydrocyanic acid, etc.
The chemical industry recommends aluminum alloys AMts, AMg2, AMgZ, AMg5 for the manufacture of vessels operating under pressure at temperatures from – 196 to +150 °C.
Containers, columns, capacitors, etc. are made from aluminum ADOO, ADO and AD1. for the production of acetic acid, sulfonation of fatty alcohols, potassium chlorate, sodium and ammonium nitrate, hydrocyanic acid, etc.
In the USA, depending on the operating conditions of chemical industry equipment, alloys of the 1xxx, 3xxx, 5xxx series are used. In some cases, to ensure the greatest strength, thermally hardenable alloys 2xxx and 7xxx with reduced corrosion resistance are used.
Tanks for storing chemical products are made of highly corrosion-resistant alloys - 1100 or 3003; high pressure vessels - made of alloys 5052 or 6063; containers, tanks and other types of equipment for storing acetic acid, high-molecular fatty acids, alcohols and other products - from alloys 3003, 6061, 6063, 5052; containers for ozone-containing fertilizer solutions made of alloys 3004; 5052 and 5454; containers for storing ammonium nitrate solutions from alloys 1100, 3003, 3004, 5050, 5454, 6061 and 6062 [3].
Electrics
Aluminum and a number of alloys based on it are used in electrical engineering due to their good electrical conductivity, corrosion resistance, low specific gravity, and, importantly, lower cost compared to copper and its conductor alloys.
Depending on the value of electrical resistivity, aluminum alloys are divided into conductive and alloys with increased electrical resistance.
The specific electrical conductivity of electrical aluminum grades A7E and A5E is about 60% of the conductivity of annealed copper according to the international standard. Technical aluminum AD0 and electrical aluminum A5E are used for the manufacture of wires, cables and buses. Low-alloy aluminum alloys of the Al-Mg-Si system AD31, AD31E are used in the electrical industry.
Aluminum alloys, which increase its strength and improve other properties, are obtained by introducing alloying additives into it, such as copper, silicon, magnesium, zinc, and manganese.
Duralumin
Duralumin (duralumin, duralumin, from the name of the German city where industrial production of the alloy began) is an alloy of aluminum (base) with copper (Cu: 2.2 - 5.2%), magnesium (Mg: 0.2 - 2.7 %) manganese (Mn: 0.2 – 1%). Subject to hardening and aging, often clad with aluminum. It is a structural material for aviation and transport engineering.
Figure 6 – Duralumin sheet
Silumin
Silumin - light casting alloys of aluminum (base) with silicon (Si: 4 - 13%), sometimes up to 23% and some other elements: Cu, Mn, Mg, Zn, Ti, Be). Parts of complex configurations are made from it, mainly in the automotive and aircraft industries.
| Figure 7 – Silumin | Figure 8 – Magnalia |
Magnalia
Magnalia are alloys of aluminum (base) with magnesium (Mg: 1 – 13%) and other elements that have high corrosion resistance, good weldability, and high ductility. They are used to make shaped castings (casting magnals), sheets, wire, rivets, etc. (deformable magnals).
In terms of breadth of application, aluminum alloys occupy second place after steel and cast iron [4].
Physical properties
Aluminum metal is characterized by high electrical conductivity, thermal conductivity, resistance to corrosion and frost, and ductility. It lends itself well to stamping, forging, drawing, and rolling. Aluminum can be welded well with various types of welding. An important property is its low density of about 2.7 g/cm³. The melting point is about 660°C. The mechanical, physicochemical and technological properties of aluminum depend on the presence and amount of impurities that worsen the properties of the pure metal. The main natural impurities are silicon, iron, zinc, titanium and copper.
According to the degree of purification, aluminum is distinguished between high and technical purity. The practical difference is the difference in corrosion resistance to certain environments. The purer the metal, the more expensive it is. Technical aluminum is used for the production of alloys, rolled products and cable and wire products. High purity metal is used for special purposes. In terms of electrical conductivity, aluminum is second only to gold, silver and copper. And the combination of low density and high electrical conductivity allows it to compete with copper in the field of cable and wire products. Long-term annealing improves electrical conductivity, while cold hardening worsens it.
The thermal conductivity of aluminum increases with increasing purity of the metal. Impurities of manganese, magnesium and copper reduce this property. In terms of thermal conductivity, aluminum is inferior only to copper and silver. Due to this property, the metal is used in heat exchangers and cooling radiators. Aluminum has a high specific heat capacity and heat of fusion. These figures are significantly higher than those of most metals. The higher the purity of aluminum, the more it is able to reflect light from the surface. The metal is well polished and anodized.
Aluminum has a high affinity for oxygen and is covered in air with a thin, durable film of aluminum oxide. This film protects the metal from subsequent oxidation and provides its good anti-corrosion properties. Aluminum is resistant to atmospheric corrosion, sea and fresh water, and practically does not interact with organic acids, concentrated or diluted nitric acid.
Use in everyday life
By studying the effect of aluminum on various food products, scientists have found that when food comes into contact with aluminum, vitamins are not destroyed. This discovery led to the widespread use of aluminum in the food industry, in the form of aluminum cookware, as well as in cosmetics and household chemicals. A variety of equipment intended for food processing in sugar, confectionery, oil mills and other industries is made from aluminum.
Figure 9 – Aluminum cookware
There is an abundance of aluminum products, both in the kitchen of a large catering establishment and in the home kitchen: meat grinders, forks, spoons, cups, basins, aluminum utensils, etc. Aluminum foil is an excellent packaging material that preserves various products well. Cooking fat, margarine, ice cream, candy and much more are packaged in aluminum foil wrappers, which is why it is also called food grade aluminum. Toothpaste is traditionally packaged in aluminum tubes. To make it convenient to use, some products, such as processed cheese, are packaged in tubes with a screw-on lid. Astronauts take food into space in these tubes. Increasingly, thin sheet food-grade aluminum is used instead of tin in the production of cans, and manufacturers are making more and more tableware from aluminum [5].
Production of household items
There are countless things in everyday life that are made from this metal. In particular, aluminum stairs are popular - they are found in almost every home and garage. Kitchen utensils, TV brackets - all these elements can be made of aluminum, not to mention smaller items.
Aluminum ladders, by the way, have confidently replaced iron ones, since the latter are very difficult to move from place to place. This once again demonstrates the advantage of this metal. The list of household items made from it could take a very long time.
Pharmaceuticals
Speaking about the versatility of aluminum, we cannot ignore an important fact: the metal from which dishes and airplanes are made is widely used to treat and prevent serious illnesses and is approved for these purposes by the World Health Organization. Of course, we are not talking about aluminum in its pure form, but about its compounds.
In 1926, it was discovered that diphtheria toxoid precipitated with alum (neutralized bacterial toxin) stimulates the production of antibodies much better than it in its pure form. Since then, aluminum salts have been most often used to enhance the effect of vaccines, since they are considered harmless to humans.
It is on the basis of aluminum that the most effective antacids are produced. Aluminum hydroxide, which neutralizes acid well, is needed to treat peptic ulcers, dyspepsia, and stomach irritation. Aluminum phosphate is suitable for the same purposes.
| Figure 10 - Medicines | Figure 11 - Deodorants |
But even those who have excellent health will benefit from a product containing aluminum, which is sold in any pharmacy, and not only that. We are talking about an antiperspirant deodorant. Even the ancient Greeks and Romans used alum to suppress secretion. Our grandmothers also used ordinary alum. Aluminum chloride was added to the first factory-made anti-sweat products, and the main agent in modern products is aluminum chlorohydrate. By the way, what the effect of their action is based on is still not known exactly [6].
Caring for aluminum cookware
If you use and care for aluminum cookware every day, it is not difficult to keep it clean.
Universal advice
To prevent pots and pans from deteriorating over time, it is better to wash them immediately after cooking. The only condition is to let it cool. If a drop of cold water falls on a hot frying pan, the pan may become deformed.
When the pan is washed immediately, no special effort is required. For washing, use warm water and mustard powder instead of chemicals. If it doesn’t work right away, and the leftover food has already dried, then fill the dishes with warm water with the addition of laundry soap or detergents and leave for 1 hour. Contaminants can be easily washed off.
How to remove blackness
With the onset of warm days, who doesn’t like to relax outside the city in nature. Smoke from the fire, barbecue, hot tea. Or fishing, fish soup, which is also cooked over a fire. Aluminum pots, saucepans, and kettles turn black after a fire. Don't be alarmed. It is not difficult to clean it. Wine vinegar or citric acid will help.
- Use a cloth soaked in vinegar or citric acid to rub the outside of the dishes and rinse thoroughly with warm water.
- If blackness has formed inside the dish, pour warm water into it, add 7-8 tablespoons of wine vinegar or squeeze out lemon juice, as much as you like.
- Boil, let cool. Drain the solution.
- Rinse with warm water using a soft sponge.
Folk remedies for washing dishes - benefits, savings or a waste of time
The dishes will become clean again.
Using hard scourers for cleaning
Under no circumstances should you clean aluminum cookware with hard sponges, much less steel or iron ones.
Firstly, they destroy the protective film of aluminum cookware, which prevents metal from getting into the food.
Secondly, hard washcloths leave wide scratches into which dirt will subsequently get clogged. It is almost impossible to wash it there.
How to clean pots and pans from burning and grease
If the dishes are very dirty and you are sorry to part with them, then use the advice on how to clean a pot or frying pan from burning and grease.
- Fill a large container with water.
- Grate or cut with a knife 1 piece of laundry soap. Pour in 300 grams of silicate glue.
- Bring the solution to a boil and place the dishes that need to be cleaned there.
- Boil over low heat with the lid closed for 1 hour.
- Remove the product from the container, being careful not to burn yourself. A soft flannel cloth or sponge can easily remove grease and carbon deposits.
- Rinse thoroughly with water.
After such a bath, your pots and pans will thank you.
How to remove scale from an aluminum surface
Using the same method that was used to remove burning and grease, you can remove scale. In addition, ammonia or vinegar will help remove it.
Dilute 8-11 drops of ammonia in a small amount of water, rub with 1/3 of a piece of laundry soap. This solution will remove a small layer of scale.
- Pour water and 5-6 tablespoons of vinegar into the container you want to descale,
- Boil the water for 10 minutes.
- Drain and rinse thoroughly with warm water.
It is not recommended to wash aluminum products in the dishwasher. They lose their shine.
How to restore shine to dishes
If your aluminum kitchen items have lost their shine and darkened, these tips will help you restore their shiny appearance.
- If it darkens inside, pour in kefir or sour milk and leave for 30-40 minutes. Rinse with warm water. Lemon or apple juice will help prevent darkening on the outside. Rub half a lemon or sour apple onto the darkened walls, leave for 1 - 3 hours and rinse with warm water.
- Instead of fruit juice, you can use 6 - 9% vinegar. The technology is the same.
- Cut the onion into several pieces, place in a bowl, add water and boil for 10 minutes.
- Rub the wetted surface with tooth powder and leave for 11 hours. Rinse thoroughly with warm water.
Your dishes will shine like new.
Cleansing with soda and sand
It is not recommended to clean aluminum cookware with these products. Aluminum is a soft metal. Soda contains alkali, and it is detrimental to the protective film. Soda will destroy it completely.
Sand in its structure consists of small particles. If you clean an aluminum surface with sand, it will leave scratches, not too big, but deep. Then dirt will get clogged there. In addition, the protective film in places of scratches will not be restored.
Advice. Do not use baking soda or sand to clean aluminum products.
Removing dark plaque
If the rules for using aluminum cookware are violated, dark spots and stains appear on its walls. Apparently, jacket potatoes, beets, and sour cabbage soup were cooked in the saucepan. It is unpleasant to use such utensils. How can I return it to its previous appearance? Regular onions will help remove this nuisance.
- Take 2 medium-sized onions and place them in the container you want to peel.
- Pour water and boil for half an hour.
Grate the laundry soap, add water and boil for 20-25 minutes.
After all procedures, thoroughly rinse the dishes with warm water and wipe dry.
Try to clean off dark spots on the walls that are not too old with an apple or lemon.
Cut the fruit in half and rub the halves into the dark areas. After this, rinse with warm water and dry.
Removing Burnt Food
To prevent food from burning, the housewife must control the cooking process, stir in time and ensure that the food does not “run away”. Then you won’t need any effort to wash the pot or pan. If it turns out that for some reason the hostess didn’t pay attention, and the food managed to burn, then don’t panic. Salt will deal with the remains of burnt food.
- Soak the pan - pour cool water into it and leave for a while.
- Drain the water and add as much salt as you like. Leave for 3-4 hours.
- Clean with a soft sponge and rinse with warm water.
If milk is burnt, activated carbon is effective.
- Grind 10 tablets into powder, pour into the bottom of the dish and leave for 1 hour.
- Pour in cool water and let the solution sit for another 30-40 minutes.
- Wash the pan with regular dishwashing detergent. Will wash off easily.
The main rule that must be followed is that if food is burnt, do not put off cleaning until later.