What it is?
Copper refining or electrolysis uses an anode that contains impure copper. It occurs due to the concentration of ore. The cathode consists of pure metal (titanium or stainless steel). The electrolyte solution consists of sulfate. Therefore, it can be argued that copper refining and electrolysis are one and the same. An electric current causes copper ions from the anodes to enter the solution and be deposited on the cathode. In this case, impurities either leave, form a precipitate, or remain in solution. The cathode becomes larger than pure copper, and the anode shrinks.
Electrolytic cells use an external source of direct current to initiate reactions that would not otherwise be spontaneous. Electrolytic reactions are used to purify plate metals on many types of substrates.
Using the electrolytic process for metal purification (copper refining, metal electrolysis):
- Since impurities can significantly reduce the conductivity of copper wires, it is necessary to clean the contaminated copper. One of the cleaning methods is electrolysis.
- When a strip of impure copper metal is used as an anode in the electrolysis of an aqueous copper sulfate preparation, the copper is oxidized. Its oxidation proceeds more easily than the oxidation of water. Therefore, metallic copper dissolves in solution in the form of copper ions, leaving behind many impurities (less active metals).
- Copper ions formed at the anode migrate to the cathode, where they are more easily reduced than water and the metal "plates" at the cathode.
It is necessary to pass sufficient current between the electrodes, otherwise a non-spontaneous reaction will occur. By carefully controlling the electrical potential, metal impurities that are active enough to oxidize copper at the anode, the substances are not reduced at the cathode, and the metal is selectively deposited.
Important! Not all metals are reduced or oxidized more easily than water. If this is the case, the electrochemical reaction requiring the least potential will occur first. For example, if we were to use electrodes, both anode and cathode, the metal potential would be oxidized at the anode, but then the water would decrease at the cathode and the aluminum ions would remain in solution.
To create electrolysis, you need to use the following copper refining method:
- Pour the copper sulfate solution into a glass.
- Place two graphite rods in the copper sulfate solution.
- Connect one electrode to the negative DC power terminal and the other to the positive terminal.
- Fill two small test tubes completely with the copper sulfate solution and place a stopper on each electrode.
- Turn on the power source and check what is happening at each electrode.
- Test any gas produced with a flaming tire.
- Record your observations and the results of your tests.
The results should be like this:
- Brown or pink solid forms appear in solution.
- There are bubbles.
- Bubbles should be colorless.
- A substance in gaseous form.
All results are recorded, after which the gas is extinguished by the tire. There is also another way to clean the metal from impurities and foreign dirt - this is fire refining of copper. We’ll tell you how this happens later, but now let’s present other options for refining metal.
Refined copper of Ukraine
| 2. Methods for producing high quality copper |
Metals are the main type of products of metallurgical production. In non-ferrous metallurgy, depending on the technology used and the composition of the resulting metals, rough and refined metals are distinguished. Commodity products supplied to the consumer for further use for their intended purpose, as a rule, are refined metals.
Crude metals are metals contaminated with impurities. Copper and nickel may contain both harmful impurities and valuable elements - satellites of the base metal. Harmful impurities worsen the properties characteristic of a given metal (electrical conductivity, ductility, corrosion resistance, etc.) and make them unsuitable for direct use. On the contrary, noble metals, selenium, tellurium, germanium, indium, bismuth and many others are of independent value, and they must be simultaneously isolated into an appropriate product, which is of great economic importance. Crude metals are necessarily subjected to purification from impurities - refining.
The quality of crude metals in some cases is established by industry standards or technical specifications that regulate the relationship between producers of crude metals and the plants to which they are supplied for refining.
The ultimate goal of copper metallurgy, like any other metallurgical production, is to obtain metals from processed raw materials in a free metallic state or in the form of a chemical compound. In practice, this problem is solved using special metallurgical processes that ensure the separation of waste rock components from the valuable components of the raw material.
Obtaining metal products from ores, concentrates or other types of metal-containing raw materials is a rather difficult task. It becomes significantly more complicated for copper and nickel ores, which, as a rule, are relatively poor and complex polymetallic raw materials. When processing such raw materials by metallurgical methods, it is necessary, simultaneously with the production of the base metal, to ensure the comprehensive separation of all other valuable components into independent commercial products with a high degree of extraction. Ultimately, metallurgical production must ensure the full use of all components of processed raw materials without exception and the creation of waste-free (dump-free) technologies.
As stated earlier, the bulk of copper ores consists of compounds of copper, iron and gangue, so the ultimate goal of metallurgical processing of these ores is to obtain a metallurgical product due to the complete removal of gangue, iron and sulfur (in the case of processing sulfide raw materials).
To obtain metals of sufficiently high purity from complex polymetallic raw materials with a high degree of complexity of their use, it is not enough to use one metallurgical process or one metallurgical unit. This task is still being implemented in practical conditions using several sequential processes that ensure gradual separation of the components of the processed raw materials.
The entire complex of applied metallurgical processes, preparatory and auxiliary operations is formed into a technological scheme of a site, department, workshop or enterprise as a whole. All enterprises involved in copper processing are characterized by multi-stage technological schemes.
The basis of any metallurgical process is the principle of converting the processed raw materials into a heterogeneous system consisting of two, three, and sometimes more phases, which must differ from each other in composition and physical properties. In this case, one of the phases should be enriched in the extracted metal and depleted in impurities, while the other phases, on the contrary, should be depleted in the main component. Differences in some physical properties of the resulting phases (density, state of aggregation, wettability, solubility, etc.) ensure good separation of them from each other by simple technological methods, for example, settling or filtration.
A modern metallurgical process must provide:
- a high degree of complexity in the use of processed raw materials;
- high specific productivity of metallurgical apparatuses;
- minimal energy costs;
- maximum use of secondary energy resources;
- using simple, cheap and easy-to-use equipment, starting up, setting up and repairing equipment;
- high degree of comprehensive mechanization and automation;
- high labor productivity;
- safe and harmless working conditions;
- elimination of harmful emissions into the atmosphere;
- maximum economic efficiency.
A high degree of complexity in the use of raw materials is the main and perhaps the most important requirement for modern technology, and it should be understood in the broadest sense.
The concept of comprehensive use of raw materials should include the highest possible extraction of all valuable components of the ore: copper, nickel, zinc, cobalt, sulfur, iron, precious metals, rare and trace elements, as well as the use of the silicate part of the ore.
Processed sulfide ores and concentrates have a fairly high calorific value and are not only a source of valuable components, but also a technological fuel. Consequently, the concept of integrated use of raw materials should also include the use of its internal energy capabilities.
Copper ores and concentrates have the same mineralogical composition, and differ only in the quantitative relationships between various minerals. Consequently, the physical and chemical bases of their metallurgical processing are exactly the same.
To process copper-containing raw materials to obtain copper metal, both pyro- and hydrometallurgical processes are used.
In the total volume of copper production, pyrometallurgical methods account for about 85% of the global production of this metal.
Pyrometallurgical technology involves the processing of feedstock (ore or concentrate) into blister copper, followed by its mandatory refining. If we take into account that the bulk of copper ore or concentrate consists of copper and iron sulfides, then the ultimate goal of copper pyrometallurgy - the production of blister copper - is achieved through the almost complete removal of gangue, iron and sulfur.
Copper production in industrial conditions can be carried out in several ways (Fig. 2.1).
The diagram shown in the figure shows that the removal of iron and sulfur can be carried out by their oxidation in three stages (roasting, smelting, converting), in two stages (smelting, converting) or in one stage. With the exception of the last option, which involves direct smelting of concentrates into blister copper, the technology for its production is characterized by a multi-stage process.
The most common technology requires the mandatory use of the following metallurgical processes: smelting for matte, converting copper matte, fire and electrolytic refining of copper.
In some cases, preliminary oxidative roasting of sulfide raw materials is carried out before smelting. Roasting is used to partially remove sulfur and convert iron sulfides and other elements into oxides that are easily slaged during subsequent smelting. As a result of firing, most of the sulfides turn into oxides, some of which volatilize in the form of oxides. The degree of removal of certain elements during the firing process, % (of their content in the feedstock):
Figure 2.1.
Schematic flow diagram of pyrometallurgical production of copper from sulfide ores
Note:
The numbers indicate possible options for processing feedstock into blister copper.
Copper matte, containing from 10...12 to 70...75% copper depending on the initial ore raw material and processing technology, is mainly processed by converting.
The main purpose of conversion is to obtain blister copper through the oxidation of iron and sulfur and some other related components. Noble metals (silver, gold), the main part of selenium and tellurium remain in the rough metal.
Blister copper, which is the final product, usually has the chemical composition given in table. 2.1.
Table 2.1.
Chemical composition of blister copper after conversion, %
Table 2.2.
Chemical composition of blister copper grades, wt. %
Blister copper is produced in the form of ingots weighing up to 1200 kg and anodes, which are used for electrolytic refining.
Copper refining is carried out by fire and electrolytic methods.
The purpose of fire refining at the preliminary (before the electrochemical) stage of production is to partially purify copper from impurities with an increased affinity for oxygen and prepare it for subsequent electrolytic refining. Using the fire refining method, they strive to remove sulfur, oxygen, iron, nickel, zinc, lead, arsenic, antimony and dissolved gases as much as possible from molten copper.
Blister copper is not suitable for direct technical use, and therefore it is necessarily subjected to refining in order to remove harmful impurities and simultaneously extract noble metals, selenium and tellurium.
Small inclusions (a few parts per million copper particles) of elements such as selenium, tellurium and bismuth can significantly impair the electrical conductivity and workability of copper - properties that are especially important to the cable industry, which is the largest consumer of refined copper. Electrolytic refining is considered the main process that produces copper that meets the most stringent electrical requirements.
The essence of electrolytic refining of copper is that a cast anode (cast, as a rule, from fire-refined copper) and cathodes - thin matrices of electrolytic copper - are alternately hung in an electrolytic bath filled with electrolyte, and a direct current is passed through this system.
In the process of electrolytic refining, two main tasks are solved:
- deep purification of copper from impurities;
- incidental extraction of related valuable components.
Anodic copper is a multicomponent alloy and usually has the chemical composition given in table. 2.3.
Table 2.3.
Chemical composition of anode copper,%
As a result of electrolytic refining, it is expected to obtain high-purity copper (99.90...99.99% Cu).
It should be noted that the higher the content of noble metals in the original copper, the lower the cost of electrolytic copper will be.
To carry out the electrolytic refining of copper, the anodes cast after fire refining are placed in electrolysis baths filled with sulfuric acid electrolyte. Between the anodes in the baths there are thin copper sheets - cathode bases.
Electrolyte is an aqueous solution of copper sulfate (160...200 g/l) and sulfuric acid (135...200 g/l) with impurities and colloidal additives, the consumption of which is 50...60 g/t Cu. The most commonly used colloidal additives are wood glue and thiourea. They are introduced to improve the quality (structure) of cathode deposits. The operating temperature of the electrolyte is 50...55 oC.
When the baths are connected to a DC network, electrochemical dissolution of copper occurs at the anode, transfer of cations through the electrolyte and its deposition on the cathode. In this case, copper impurities are mainly distributed between sludge (solid sediment at the bottom of the baths) and the electrolyte. In Fig. 2.2. A diagram of the electrolytic refining process is shown.
Figure 2.2.
Electrolytic refining process diagram
As a result of electrolytic refining, the following is obtained: cathode copper; sludge containing precious metals; selenium; tellurium and contaminated electrolyte, part of which is sometimes used to produce copper and nickel sulfate. In addition, due to incomplete electrochemical dissolution of the anodes, anodic residues (anode scrap) are obtained.
Electrolytic refining is based on the difference in the electrochemical properties of copper and the impurities it contains.
Copper belongs to the group of electropositive metals, its normal potential is +0.34 V, which allows the electrolysis process to be carried out in aqueous sulfuric acid solutions.
Impurities are divided into four groups based on their electrochemical properties:
- Group 1 - metals more electronegative than copper (Ni, Fe, Zn);
- Group 2 - metals located close to copper in the voltage range (As, Sb, Bi);
- Group 3 - metals more electropositive than copper (Au, Ag, platinum group);
- Group 4 - electrochemically neutral chemical compounds (Cu2S, Cu2Se, Cu2Te, etc.).
The mechanism of electrolytic refining of copper includes the following elementary stages:
- electrochemical dissolution of copper at the anode with the removal of electrons and the formation of a cation: Cu - 2e -> Cu2+;
- transfer of the cation through the electrolyte layer to the cathode surface;
- electrochemical reduction of copper cation at the cathode: Cu2+ - 2e -> Cu;
- introduction of the resulting copper atom into the crystal lattice (growth of cathode deposit).
Impurities of the first group, which have the most electronegative potential, are almost completely transferred to the electrolyte. The only exception is nickel, about 5% of which is deposited from the anode into sludge in the form of a solid solution of nickel in copper. According to Nernst's law, solid solutions become even more electropositive than copper, which is the reason for their transition to slurry.
A special behavior in comparison with the listed groups of impurities is demonstrated by lead and tin, which in terms of electrochemical properties belong to group 1 impurities, but in terms of their behavior during the electrolysis process can be classified as impurities of groups 3 and 4. Lead and tin form lead sulfate PbS04 and metatinic acid H2Sn03, insoluble in sulfuric acid solution.
During the electrolysis of copper, electronegative impurities are practically not deposited on the cathode and gradually accumulate in the electrolyte. At a high concentration of metals of the first group in the electrolyte, electrolysis can be significantly disrupted.
The accumulation of iron, nickel and zinc sulfates in the electrolyte reduces the concentration of copper sulfate in the electrolyte. In addition, the participation of electronegative metals in current transfer through the electrolyte enhances the concentration polarization at the cathode.
Electronegative metals can enter cathode copper mainly in the form of intercrystalline inclusions of solution or basic salts, especially when they are significantly concentrated in the electrolyte. In the practice of electrolytic refining of copper, it is not recommended to allow their concentration in solution to exceed the following values, g/l: 20 Ni; 25 Zn; 5 Fe.
Impurities of group II (As, Sb, Bi), having electrode potentials close to copper, are the most harmful from the point of view of the possibility of cathode contamination. Being somewhat more electronegative compared to copper, they completely dissolve at the anode to form the corresponding sulfates, which accumulate in the electrolyte. However, the sulfates of these impurities are unstable and undergo significant hydrolysis, forming basic salts (Sb and Bi) or arsenous acid (As). Basic antimony salts form flakes of gelatinous sediments (“floating” sludge) floating in the electrolyte, which partially capture arsenic.
Impurities of arsenic, antimony and bismuth can enter cathode deposits either electrochemically or mechanically as a result of the adsorption of fine particles of “floating” sludge. Thus, group 2 impurities are distributed between the electrolyte, cathode copper and sludge. The maximum permissible concentrations of group 2 impurities in the electrolyte are, g/l: 9 As; 5 Sb and 1.5 Bi.
Impurities that are more electropositive compared to copper (group 3), which include noble metals (mainly Au and Ag), in accordance with their position in the voltage series, should pass into sludge in the form of a finely dispersed residue. This is confirmed by the practice of electrolytic refining of copper.
The transition of gold into sludge is more than 99.5% of its content in the anodes, and silver - more than 98%. The slightly lower transition of silver into slurry compared to gold is due to the fact that silver can dissolve in a small amount in the electrolyte and then separate from the solution at the cathode. To reduce the solubility of silver and transfer it to sludge, a small amount of chlorine ions is introduced into the electrolyte.
Chemical compounds (group 4 impurities) behave similarly to electropositive impurities during the electrolysis of copper. Although, in principle, chemical compounds can be oxidized at the anode and reduced at the cathode, which is used in special processes, under the conditions of electrolytic refining of copper the anodic potential is not enough for their oxidation. Therefore, during the electrolysis of copper, they do not participate in the electrode processes and, as the anode dissolves, they fall off to the bottom of the bath. More than 99% of selenium and tellurium pass into sludge in the form of selenides and tellurides.
Thus, as a result of electrolytic refining of anode copper, all impurities contained in it are distributed between cathode copper, electrolyte and sludge.
Current density is the most important parameter of the electrolysis process. The current density during electrolysis is usually chosen from 220...230 to 300 A/m2 of cathode area, and the total energy consumption is from 1800 to 4000 MJ/t of anodes (electricity 200...300 kW*h/t of copper).
The electropositive potential of copper allows copper to be isolated at the cathode from acidic solutions without fear of hydrogen evolution. The introduction of free sulfuric acid into the electrolyte along with copper sulfate significantly increases the electrical conductivity of the solution. This is explained by the greater mobility of hydrogen ions compared to the mobility of large cations and complex anionic complexes.
Depending on the electrolysis system, thin copper, titanium and steel sheets are used as a cathode base (matrix). Anodes are usually cast weighing 250...360 kg. The duration of anode dissolution is from 20 to 28 days.
During this time, two or three cathodes are removed, the mass of each of which is 100...150 kg. Cathodes are the end product of electrolytic refining of copper.
During the electrolysis process, dendrites can form on the surface of the cathode, which reduces the distance between the cathode and the anode at a given location. A decrease in the interelectrode distance leads to a decrease in electrical resistance, and, consequently, to a local increase in current density. The latter, in turn, causes accelerated deposition of copper on the dendrite and its accelerated growth. Once dendrite growth begins, it can eventually lead to a short circuit between the cathode and anode.
Cathodes must be dense and non-fragile. There should be no dendritic growths of porous copper on the surface of the cathode. The presence of growths grown into the body of the cathode is allowed on cathodes made of copper grades M0ku, M0k and M1k. The surface of the cathodes and cathode ears must be clean, well washed from the electrolyte, and must not have deposits of copper and nickel sulfates.
To avoid surface contamination, cathodes made of copper grades M00k are supplied packaged in wooden boxes or metal containers.
The dimensions of the cathodes in accordance with GOST 546 are agreed upon between the manufacturer and the consumer. The mass of the cathode ranges from 50 to 120 kg and above. Cathodes contain from 9 to 20 cm3 of gases per 100 g of substance. For example, gas-containing copper cathodes of grade M0k contain, % (by mass) O2 - 1...9*10-3; H2 - 2...7, 5*10-4 and N2 - 0...7*10-3.
The problem of the appearance and structural state of the cathode complicates and increases the cost of electrochemical refining technology. In most cases, cathodes are not directly suitable for the production of high-quality rolled products. Therefore, manufacturers melt a significant part of cathode copper into ingots, which are called wirebars (blanks for rolling and drawing). Using this sophisticated technology, oxygen-free copper is obtained for the production of thin wire.
Electrolytic refining of copper allows for the complete extraction of gold, silver, platinum and rare metals (Se, Te, Bi, etc.) and provides sufficiently deep purification from harmful impurities. The cost of the recovered copper satellites usually covers the entire cost of refining, so this process is very economical.
Gold and silver are extracted during the processing of copper ores with great completeness and along with copper without organizing special processing (except for the necessary processing of rich electrolysis sludge). Therefore, the maximum involvement of gold-containing raw materials (for example, quartzites) in associated processing together with copper ores is economically very effective and is used to the maximum.
The question arises: why, if the technological scheme contains electrolytic refining, which can clean copper from all harmful impurities and extract valuable components, fire refining is also included? Practice and economic calculations have clearly proven that two-stage refining of blister copper is cheaper than its direct electrolytic purification.
This is due to a lower yield of anode scrap, the production of richer slurries, less contamination of the electrolyte, lower energy costs and a number of other factors, ultimately leading to lower overall costs and higher extraction of both copper itself and its valuable satellites into marketable products. At the same time, this leads to an improvement in the quality of commercial copper.
More than 95% of blister copper smelted is currently subjected to two-stage refining. First, copper is refined using a fire (oxidative) method, and then electrolysis is carried out. In some cases, when copper does not contain noble metals, its purification is limited to fire refining. The typically achieved copper purity after traditional fire refining is 99.9 wt% Cu. The red copper obtained in this case is used for rolling into sheets and for the preparation of a number of alloys.
There are three possible options for organizing the refining of blister copper in industrial conditions:
- Both stages of refining are carried out at the same enterprise where blister copper is smelted. In this case, copper is supplied to fire refining in a molten state.
- Both stages of refining are carried out at special refineries, to which blister copper is supplied in ingots weighing up to 1500 kg. This technology requires re-melting of the crude metal, but allows on-site processing of anode residues from the electrolysis process and technological waste.
- Fire refining of liquid blister copper is carried out at copper smelters, and electrolysis of anodes is carried out centrally at special enterprises. This option for refining blister copper is typical, in particular, for the production of refined copper in the USA.
Thus, the two-stage production technology “fire refining - electrolysis” will make it possible to obtain high-quality products - cathode copper, but at the same time it has a number of significant limitations. The main limitation is related to the technical and economic indicators of the process, which is focused on the use of primary copper obtained from ore.
The presence of precious and rare metals in the ore and their extraction at the refining stage ensure an acceptable cost of the final product (see Fig. 2.1).
If the material that goes for electrolysis contains little or no impurities, the cost-effectiveness of copper cathode production becomes problematic.
For example, in Ukraine, copper cathode is produced by the Konstantinovsky Metallurgical Plant (Donetsk region). Recently, it has reduced the production of copper anodes by 18% and, with a design capacity of 2.4 thousand tons of copper per year, provides only its own production with copper. It is not able to satisfy the growing needs of Ukrainian industry for high-quality copper.
There are other restrictions that hinder the expansion of copper cathode production in countries that do not have industrial reserves of copper ores:
- Copper is obtained in the solid state (cathodes), which requires additional energy costs for the production of the final product.
- initial cathode matrices are required - rolled polished plates with a thickness of 3...6 mm;
- significant production areas are required to accommodate electrolysis baths;
- powerful water treatment facilities are needed to regenerate and neutralize the acid electrolyte;
- the environmental load on the environment, pollution from acid fumes and other harmful substances and wastewater increases.
Accordingly, when developing a general technology for producing high-quality copper, more attention had to be paid to debugging the electrolysis technology to ensure maximum extraction of valuable metals, removal of harmful impurities, and reduction of man-made load on the environment. Fire refining technology was considered as an intermediate one, the task of which is to obtain an intermediate product (anodes) with an approximately specified composition.
The increase in global volumes of copper produced, problems arising with the extraction and processing of ore, have led to the need to expand the use of fire refining as the last technological stage in the production of high-quality copper.
In this case, the initial raw material will not be blister copper, but secondary copper-containing raw materials. As a result of fire refining, it is necessary to obtain not a semi-product (anodes), but finished high-quality copper, which is used for the manufacture of products required by the customer.
It is impossible to achieve a fundamental change in the level of impurities in fire-refined copper without a deep theoretical analysis of the possibilities of oxidative refining. Simply using existing technological developments in this area is impossible due to fundamental differences in the composition of the initial secondary raw materials. The main difference between the raw materials available in Ukraine and similar secondary raw materials in other countries with a developed copper smelting industry is the significant share of household waste and the unpredictable ratio of the content of various impurities.
Copper smelters abroad use higher quality secondary raw materials with narrow limits for composition changes. Accordingly, the requirements for their technological process are less stringent. Ukrainian enterprises work on low-quality raw materials, but the technologies used must ensure the production of the same high-quality copper and competitive products from it.
In Fig. Figure 2.3 shows a typical view of a batch of scrap metal received for processing at the Artemovsk Non-Ferrous Metals Processing Plant.
Figure 2.3.
Type of batch of scrap metal received by OJSC "AZOTsM".
Let's consider the main indicators of the advanced fire refining technology, which is used by one of the leading European companies - La Farga Lacambra in Spain. From scrap and waste copper (mainly electrical) this company produces liquid copper of the FRTP and Cu-DHP grades according to BS EN 12163:1998, which is transported via chutes to the Properzi casting and rolling complex for the production of copper rod, or is transported using a ladle to the gas mixer of a machine for vertical casting of round blanks for further pressing.
In table 2.4 and 2.5 present comparative data on the quality of raw materials and requirements for them for Ukrainian manufacturers and the company La Farga Lacambra.
Table 2.4.
Comparison of the quality of raw materials from Ukrainian manufacturers and La Farga Lacambra
Table 2.5.
Comparison of quality requirements for raw materials of Ukrainian manufacturers and La Farga Lacambra
The La Farga Lacambra fire refining technology has a number of significant disadvantages that do not allow metallurgical enterprises in Ukraine to use it even with the most modern equipment:
- The inability to remove nickel with a content level of 600...900 ppm and tin - 800...900 ppm from the copper melt does not allow scrap and waste collected in Ukraine to be processed using this technology.
- There is no possibility of involving in production tinned and soldered scrap and waste, as well as scrap of bronze and brass with a copper content of 92%.
- There is no removal of non-metallic impurities during the preparation of raw materials for smelting, which leads to additional losses of copper with slags that form these non-metallic impurities.
- There is no analysis of gas composition. This does not allow intensifying the recovery process (oxygen removal) from the copper melt, ensuring safe working conditions and increasing the durability of equipment (due to a possible increase in CO concentration in the furnace atmosphere and chimneys).
- Low durability of the reverberatory furnace lining, which is associated with the aggressiveness of fluxes and the use of “wet” shotcrete during routine repairs.
- Insufficiently long overhaul period of the furnace operation, associated with partial removal of the dust-flux mixture, its adhesion to the smoke gate and its rapid accumulation in the deposition chamber.
- The inability to withstand the daily production cycle of the complex, since the capacity of the fire refining furnace of JSC AZOTsM is much larger than that of La Farga Lacambra, and the productivity of the equipment and loading processes are not productive enough.
To meet the needs of Ukrainian industry, more powerful production is required and a noticeably larger range of grades of copper and alloys made from it than at La Farga Lacambra and other well-known manufacturers, therefore, the technology must be flexible and easily adaptable depending on the wishes of the consumer and the available raw materials.
Taking these reasons into account, Ukrainian enterprises that produce copper from recycled materials need to develop fire refining technology, which not only eliminates the disadvantages of analogues, but also has greater efficiency.
| 2. Methods for producing high quality copper |
Methods for refining copper - how else can chemical stripping of the desired metals occur?
Since electrolysis is the action of sulfates and current, what is the electrolytic method for obtaining pure products? Completely different things, although the names sound similar. However, electrical refining of copper involves the use of acids. We can say that this is oxidation of the metal, but not quite.
Pure products are important for making electrical wire because the electrical conductivity of copper is reduced by impurities. These impurities include valuable metals such as:
- silver,
- gold;
- platinum.
When they are removed by electrolysis and restored in the same way, the amount of electricity consumed is enough to supply electricity to dozens of homes. The purified component saves energy, powering even more homes in less time.
In electrolytic refining, an impure composition is made from an anode in an electrolytic bath of copper sulfate - CuSO4 and sulfuric acid H2SO4. The cathode is a sheet of very pure copper. As current is passed through the solution, positive copper ions, Cu2+, are attracted to the cathode, where they take up electrons and are deposited as neutral atoms, thereby creating more and more pure metal at the cathode. Meanwhile, the atoms in the anode give up electrons and dissolve in the electrolyte solution as ions. But impurities in the anode do not go into solution because the silver, gold and platinum atoms do not oxidize (become positive ions) as easily as copper. This way, the silver, gold and platinum simply fall from the anode to the bottom of the tank, where they can be cleaned.
But there is also electrolytic refining of copper when tanks are used:
- Electrolytic treatment tanks are a separate workshop in industrial production. The anode plates are suspended by "handles" in the electrolytic copper purification tank. Pure copper cathode sheets suspended on solid rods are inserted into the same tank, one sheet between each anode. When electric current is passed from the anodes through the electrolyte to the cathodes, the copper from the anodes moves into the solution and lands on the starter sheet. Impurities from the anodes settle to the bottom of the tank.
- Injection molding machine with copper anodes (plates). It will be smoothly converted into anode plates into molds. After pre-treatment, tin, lead, iron, and aluminum are removed. Next, the copper material begins to be charged into the furnace, followed by the smelting process.
- Once the impurities are removed, deslagging and a reduction phase with natural gas follows. The reduction is aimed at removing free oxygen. After recovery, the process ends with casting, where the final product is cast in the form of copper anodes. The same machine can be used to cast these anodes during component recycling or to recycle anodes for scrap metal in an electrolysis copper smelter.
- Clean cathode sheets. The inoculant anodes removed from the refining furnace are converted into 99.99% pure electrolytic copper through the electrolysis process. During electrolysis, copper ions leave an unclean copper anode and, since they are positive, migrate to the cathode.
From time to time, pure metal is scraped off the cathode. Impurities from the copper anode, such as gold, silver, platinum and tin, collect at the bottom of the electrolyte solution and are deposited as anode slime. This process is called electrolytic copper production and refining.
Cathode production
Before loading the anodes, the series is turned off and the circulation of the electrolyte is stopped. Check the electrolyte level in the baths, remove excess with a siphon. Dry wood or vinyl plastic bars and well-washed triangular copper busbars are placed on the sides of the bathtubs. The anodes are weighed and loaded into melts. On one side, the anodes rest on an insulating block, on the other hand, on a busbar. The distance between the anodes is set using a measuring rod. The anode in the bath must be installed strictly vertically. To quickly warm up the electrolyte, circulation is turned on before the end of the anode loading operation.
The cathode bases before the curtain must be well straightened and have firmly riveted ears. Before the curtain, the anode contacts and triangular busbars are blown with steam. The crowbars on which the cathode bases are suspended are first washed with hot water acidified with sulfuric acid. The crowbars are pushed through the ears of the cathode bases and, when hanging the cathodes, they are installed with one end on the busbar and the other on the insulating block.
Rice. 97. Tools and devices used in electrolysis: 1 - card tape for stripping contacts; 2 — hook for adjusting the position of the anode in the bath; 3 - hook for eliminating short circuits of the anode and cathode from below; 4 — portable current indicator (electric probe); 5 - a wooden pawn is placed to straighten the position of the anode in the bath; 6 — crowbar for straightening the cathode; 7 - chisel for removing cathode bases
Before turning on the series, check that there are no short circuits between the cathode bases and the anodes or bath walls, as well as the correct location of the crowbars on the tires. The top edges of the anodes and cathode bases should be below the electrolyte level. The series is turned on when the electrolyte temperature becomes at least 40°. The temperature of the electrolyte is measured every hour. After 3-6 hours. The cathodes are straightened, for which the cathodes are removed individually and straightened, and then hung again. The instrument used in electrolysis is shown in Fig. 97. Short circuits are detected by an electrical probe, they are indicated by a drop in voltage, heating of the crowbars and contact points. An increase in voltage indicates contact contamination. Contacts are cleaned with card tape. Eliminating short circuits and cleaning contacts improves the electrolysis process and increases current utilization (current efficiency).
The anodes are kept in electrolyte baths for 22-30 days. Cathode growth lasts 6-15 days. Thus, one batch of anodes is enough to form 2-4 times more cathodes. When the series is operating in the third or fourth period, the worn-out anodes are replaced with a substation—usable anodes selected from the anode residues. The undergrowth is pre-straightened.
To ensure copper build-up on the ears, in order to prevent cathode breakage, the electrolyte level in the baths is adjusted by installing lead rings in the bath trays. The upper edges of the cathodes should be immersed in the electrolyte by 5-10 mm. The cathode deposit of copper must have a fine-crystalline structure and a smooth surface.
Cathodes from the series are removed by an overhead crane using a device for capturing cathodes - a “harrow”.
For some time, the cathodes are kept above the bath for the electrolyte to soak and then sent for washing with water or steam treatment to remove the electrolyte and, after control, transported to the warehouse. Two cathodes are taken from each series as a sample for chemical analysis. Before unloading the anode residues, the supply of electrolyte to the bath is stopped. The anode residues are washed from sludge, sorted, and the unused ones are returned to the electrolysis cycle as a refill. The spent anode residues are sent to the smelting shop. After unloading the anodic residues, the old insulating bars are removed.
Requirements for the quality of copper cathodes
- The surface of the cathodes should not have accumulations of pronounced mushroom-shaped dendritic growths, as well as large growths of porous copper on the edges. Lumps in the form of individual spherical growths that have grown into the body of the cathode and do not separate from it with light blows are not a rejection sign. Cathodes obtained in the form of a fine-crystalline dense deposit and having the most even outer surface usually meet high requirements and, as a rule, contain the least amount of hydrogen and oxygen.
- Cathodes should not be brittle and should not break from impacts. A good quality copper cathode has a smooth cut and a dense layer of copper that does not break when bent. The fragility of cathodes is associated with gas porosity and layers of foreign inclusions in the deposited layer. When bent, this layer crumbles and lags behind the mother sheet; the cut is rough.
- The surface of the cathodes and cathode ears must be clean, well washed from the electrolyte and must not have a deposit of copper sulfate.
Obtaining fossils - what types exist and are they all necessary in practice?
Another method of cleaning metal is somewhat different. There is also fire and electrolytic copper refining, when one process immediately follows the other. An important “separating” step is concentration or concentration. Once concentration is complete, the next step in creating the finished product is fire refining of the copper.
This usually occurs near a mine, processing plant or smelter. Copper refining gradually removes unwanted material and concentrates the copper to a purity of up to 99.99% Grade A. The details of the refining process depend on the type of minerals the metal is associated with. Copper ore, rich in sulfides, is processed using the pyrometallurgical process.
Processing and pyrometallurgy:
- In pyrometallurgy, copper concentrate is dried before heating in a furnace. Chemical reactions that occur during the heating process cause the concentrate to separate into two layers of material: a matte layer and a slag layer. The matte layer at the bottom contains copper, and the slag layer at the top contains impurities.
- The slag is discarded and the matte layer is recovered and transferred to a cylindrical vessel called a converter. Various chemicals are added to the converter which react with the copper. This results in the formation of converted copper called "blister". Once deposited, it is recovered and then subjected to another process called fire refining.
- In fire refining, air and natural gas are purged to remove remaining sulfur and oxygen, resulting in the purified composition being processed into a cathode. The metal is cast into anodes and placed in an electrolyzer. After charging, pure copper is collected at the cathode and removed as a 99% pure product.
Processing and hydrometallurgy:
- In hydrometallurgy, copper concentrate is processed through one of several processes. The least common method is carburization, where metal is deposited onto scrap metal in an oxidation-reduction reaction.
- A more widely used purification method is solvent extraction and electrolysis. This new technology became widespread in the 1980s, and approximately 20% of the world's copper is now produced this way.
- Solvent extraction begins with an organic solvent that separates the metal from impurities and unwanted materials. Sulfuric acid is then added to separate the copper from the organic solvent to form an electrolytic solution.
- This solution is then subjected to an electrolysis process, which simply puts the copper in solution onto the cathode. This cathode can be sold as is, but can also be made into rods or starting sheets for other electrolysers.
Mining companies may sell copper in concentrate or cathode form. As mentioned above, the concentrate is most often refined at a location other than the mine site. Concentrate manufacturers sell concentrate powder containing 24 to 40% copper to copper smelters and refineries. The terms of sale are unique to each smelter, but in general the smelter pays the miner approximately 96% of the cost of containing the copper in the concentrate, less processing fees and refining costs.
Typically, smelters collect tolls, but they may also sell the refined metal on behalf of the miners. Thus, all the risk (and reward) from fluctuations in copper prices falls on the shoulders of resellers.
Preparation of copper oxide
Copper (II) oxide CuO is a black crystal that undergoes crystallization in a monoclinic system. The density of the compound is 6.51 g/cm3, and it melts at a temperature of 1447 ° C under high pressure conditions. As a result of heating to 1100°C, copper (I) oxide is released:
- 4CuO = 2Cu2O + O2.
Copper oxide does not dissolve in water and does not react with it. It has weak amphoteric properties with a predominance of basic ones. Reacts with aqueous solutions of ammonia to form tetraammine copper (II) hydroxide:
- CuO + 4NH3 + H2O = [Cu(NH3)4](OH)2.
It also easily reacts with dilute acids, releasing salt and water:
- CuO + H2SO4 = CuSO4 + H2O.
The result of the fusion of copper oxide with alkalis is the formation of cuprates:
- CuO + 2KOH = K2CuO2 + H2O.
Pure copper oxide can be obtained by reduction with hydrogen, carbon monoxide and active metals:
- CuO + H2 = Cu + H2O
- CuO + CO = Cu + CO2
- CuO + Mg = Cu + MgO.
The reaction for producing copper oxide by calcination of copper (II) hydroxide at a temperature of 200°C:
- Cu(OH)2 = CuO + H2O
Copper oxide can also be obtained through the oxidation of copper metal in air at a temperature of 400–500°C:
- 2Cu + O2 = 2CuO.
Fire refining – how dangerous is it?
The most popular fire refining cannot be without danger, but currently the processing method is used in most industrial enterprises. Separately, it is worth describing the technology for refining blister copper.
Blister copper is already almost pure (more than 99% copper). But for today's market this is not very “clean”. The metal is further purified using electrolysis. In industrial production, a method called fire refining of blister copper is used. Ink copper is cast into large slabs to be used as anodes in the electrolyser. Electrolytic additional refining produces the high-quality, high-purity metal required by the industry.
In industry this is done on a massive scale. Even the best chemical method cannot remove all impurities from copper, but electrolytic refining can produce 99.99% pure copper.
- Anode blisters are immersed in an electrolyte containing copper sulfate and sulfuric acid.
- Between them are clean cathodes, and a current of more than 200 A passes through the solution.
Under these conditions, copper atoms dissolve from the unclean anode to form copper ions. They migrate to the cathodes, where they are deposited back as pure copper atoms.
- At the anode: Cu(s) → Cu2 + (aq) + 2e-.
- At the cathode: Cu2 + (aq) + 2e- → Cu(s).
When the switch closes, the copper ions at the anode will begin to move through the solution to the cathode. Copper atoms have already given up two electrons to become ions, and their electrons can move freely in the wires. Closing the switch pushes the electrons clockwise and causes some copper ions to precipitate into the solution.
The plate repels ions from the anode to the cathode. At the same time, it pushes free electrons around the wires (these electrons are already distributed throughout the wires). The electrons in the cathode recombine with copper ions from the solution, forming a new layer of copper atoms. Gradually, the anode collapses and the cathode grows. Insoluble impurities in the anode fall to the bottom in sediment. This valuable bioproduct is removed.
Gold, silver, platinum and tin are insoluble in this electrolyte and therefore are not deposited on the cathode. They form valuable “sludge” that accumulates under the anodes.
Soluble iron and nickel impurities dissolve in the electrolyte, which must be continually purified to prevent excessive deposition on the cathodes, which will reduce the purity of the copper. Recently, stainless steel cathodes have been replaced by copper cathodes. Identical chemical reactions occur. Periodically, the cathodes are removed and the pure copper is purified. Electrolytic production and refining of copper under these conditions is quite common in non-ferrous metal processing plants.
Production of cathode bases
Cathode bases are made from master sheets obtained by electrolytic deposition of copper on matrices. Before use, new matrices are washed with water acidified with sulfuric acid, wiped, dried, and then treated with a solution containing 6-8 g/l sodium sulfide. Before installation, they are thoroughly wiped with a rag, after which an even layer of lubricant is applied, consisting of 1 part fatty grease and 6-8 parts kerosene.
Copper is deposited electrolytically on both sides of the matrices in a layer of 0.25–0.7 mm. To facilitate stripping of the mother sheets, the matrices have grooves along the edges, on which sediment forms in the form of a film that can be easily cleaned off with a chisel. Recently, matrices began to be made with folds from an acid-resistant plastic mass - faolite. If there are folds, copper deposits do not form at the edges of the matrices, which greatly facilitates the stripping of the mother sheets.
Matrices that are bent or with bent crowbars are not allowed to be planted in bathtubs.
In matrix series baths, an electrolyte with a high content of copper sulfate and a reduced content of sulfuric acid is used. The electrolyte used is purer: with less impurities, with an increased amount of additives that improve the structure of the sediment. The electrolyte temperature should not exceed 56°, since an increase in temperature causes the dissolution of the lubricant applied to the matrices.
Anodes made of blister copper in matrix baths last 13-16 days. Duration of extension of mother sheets is 24 hours. Typically, an electrolyte of the following composition is used: copper sulfate 130-160 g/l, free sulfuric acid 100-160 g/l. As colloidal additives, wood glue or gelatin and chlorine ion in the form of hydrochloric acid are introduced into the electrolyte composition. The current density should not be higher than 170 A/m2.
Periodically check that the anodes and matrices are positioned correctly in the baths and that there are no short circuits.
Matrices with accumulated sediments are removed using a “harrow”. At the same time, it is allowed to remove no more than half of the matrices from the bath; the number of such baths should be no more than four in a series. The removed matrices are kept above the baths to drain the electrolyte from them, after which they are fed for washing and then to special machines for stripping off the sheets. On the machines, the dies are sequentially fed onto a rotating frame, which allows the sheets to be removed from both sides of the dies without manual turning. The sheets are cut according to the marks, and if there are folds, the stripping is carried out by tearing off the sheets at the top of the matrices. After riveting the ears and straightening on rollers, the cathode bases are ready for use.
The matrices are cleaned again, bent ones are straightened, then lubricated and sent again to the electrolytic production of master sheets.
Electrochemical option for metal purification
Fire cleaning can be called chemical cleaning, because in this process a chemical reaction occurs with other substances and impurities. An example of an oxidative reaction was given above. All types and methods of extracting pure copper are similar, as is electrochemical copper refining, where identical tactics are used, but in different sequences.
The by-product itself becomes a chemical auxiliary element:
- Sodium hydroxide.
- Chlorine.
- Hydrogen.
This is the cheapest way to obtain expensive raw materials without spending money on an alternative component extraction system. In addition, valuable metals are mined, which are noble in composition and valuable in the industrial invention of electrical appliances.
Copper ores and their deposits
At the moment, obtaining Cu is considered economically profitable and feasible even if it is contained in the rock at least 0.3%.
Most often, the following rocks are mined in nature today to extract copper industrially:
- bornites Cu5FeS4 - sulfide ores, otherwise called copper purple or variegated pyrite and containing about 63.3% Cu;
- chalcopyrites CuFeS2 - minerals of hydrothermal origin;
- chalcocite Cu2S containing more than 75% copper;
- cuprites Cu2O, often also found in places of native copper deposits;
- malachites, which are carbonated copper greens.
The largest copper ore deposit in Russia is located in Norilsk. Also, such rocks are mined in large quantities in some places in the Urals, Transbaikalia, Chukotka, Tuva and the Kola Peninsula.
Copper oven – metal cooking industry
The copper fire refining furnace is specially designed and is capable of processing copper scrap into liquid metal with controlled impurity content. It is designed for pyrometallurgical processing of scrap using economical and environmentally friendly technology. The main technology proposed for the production of molten copper is suitable for the production of copper stick, strip, billet or other copper products using scrap as raw material (Cu > 92%).
The capacity of the combustion and treatment systems was calculated for a treatment cycle (from charging to recovery) of 16-24 hours, depending on the type of scrap. Copper refining furnaces have special design and functions:
- The furnace body is made of steel segments and rigid section-type structures.
- The oven is lined with refractory material from the inside.
- It is equipped with a hydraulic station operating in the tilting mode of the furnace with two speeds: creep speed when tilting for casting and high speed during movement, which does not require much precision.
- Operations are carried out using two hydraulic cylinders installed on the bottom of the furnace. A special device returns the oven to a horizontal position during emergency power outages.
- The material loading hatch is located in the side of the furnace. It is closed by a door driven by a hydraulic cylinder.
- The furnace is equipped with cooled lances for copper oxidation and reduction operations.
There is also one universal burner that consumes both liquid and gaseous fuel.
Preparation of copper sulfide
Copper(II) sulfide or copper monosulfide - CuS, is an inorganic binary compound of divalent copper with sulfur. It is of the correct color, does not dissolve in water, and is also acidic in dilute solutions. In nature, it can be found in the form of the rare mineral covellite. Copper sulfide is obtained through direct interaction of elements, as well as as a result of the exchange reaction of divalent copper salts with water-soluble sulfides.
- Na2S+CuSO4=CuS+Na2SO4
- CuCl2 + H2S —> CuS + 2HCl
- 2CuS + H2 —>Cu2S + H2S. This reaction occurs under high temperature conditions of 600 to 700 oC
Preparation by the dry method gives copper sulfide the ability to conduct electric current. When the thermometer reaches 400 °C, noticeable decomposition of sulfide is observed.
Oxidative refining in industry
The copper oxidation operation is carried out after completion of the smelting of the feedstock. The process is carried out by injecting compressed air into the melt through tuyeres. The resulting slag is removed manually from the surface of the melt using a special rake and dumped into a container. The slag contains copper, impurities, lead, tin, etc. The reduction process must be carried out to remove oxygen from the melt and reduce the copper oxides. The operation is performed by injecting natural gas into the melt.
From the furnace, exhaust gases are fed into the gas cleaning system and pass through a dust collector, which captures coarse dust. The collector is equipped with a ventilation pipe in the event of an emergency release of gas into the atmosphere. The fire cleaning furnace operates in continuous mode. The operating cycle of the technological process includes:
- loading of raw materials;
- oxidation, slag formation, reduction;
- loading of refined metal.
The entire subsequent process is called oxidative refining of copper. It cannot be separated from the general purification process, since it is part of the entire method of obtaining pure metal. Once the required parameters have been resolved, the copper melt is used for the next process.
Sludge removal
The sludge that accumulates in the baths is removed at least once every 2 months. The baths are disconnected from the circulation system; After settling and removing the main part of the electrolyte, the sludge pulp is removed through a hole in the bottom of the bath or by siphoning, and sometimes scooping into a special sludge bucket. After filtering the sludge pulp, the clarified electrolyte is fed into the circulation system, and the sludge separated from the liquid is sent for processing to the sludge department to extract precious and other metals.
Iodide refining of non-ferrous metals
Copper(II) ions oxidize iodide ions to molecular iodine, and in this process are themselves reduced to copper(I) iodide. The original mixed brown mixture separates into an off-white precipitate of copper(I) iodide in iodine solution. This reaction is used to determine the concentration of copper (II) ions in solution. If you add a specified volume of a solution containing copper(II) ions to a flask and then add an excess of potassium iodide solution, you will obtain the reaction described above.
2Cu2+ + 4I- → 2CuI (s) + I2 (aqueous solution)
You can find the amount of iodine released by titrating with sodium thiosulfate solution.
2S2O2-3 (solution) + I2 (solution) → S4O2-6 (aqueous solution) + 2I- (aqueous solution)
When the sodium thiosulfate solution is run from the burette, the color of the iodine disappears. When it's almost all gone, add starch. The entire copper iodide refining reaction will be reversible with iodine to produce a deep blue starch-iodine complex that is much easier to see.
Add the last few drops of sodium thiosulfate solution until the blue color disappears. If you trace the proportions through the two equations, you will find that for every 2 moles of copper(II) ions you had to start with, you need 2 moles of sodium thiosulfate solution. If you know the concentration of the sodium thiosulfate solution, it is easy to calculate the concentration of copper(II) ions. The result of this attempt is to obtain a simple copper(I) compound in solution.
Preparation of copper glycerate
A qualitative reaction to detect the presence of glycerol in solutions is carried out in the presence of copper (II) sulfate and sodium hydroxide solution. As a result of the reaction, copper glycerate is formed - a complex compound with a blue-cornflower blue hue. The chemical reaction is carried out as follows:
- a solution of sodium hydroxide is added to a solution of copper (II) sulfate, resulting in the solution turning blue. Thus, we observe the precipitation of copper (II) hydroxide
- after that, add a few ml of glycerin and mix the solution. The resulting precipitate dissolves to form an indigo-colored complex compound. This is copper glycerate.
The equation is as follows: CH2OH-CHOH-CH2OH + Cu(OH)2—> Cu(-O-CH2-CH-O-)-CH2OH
Phosphorous treatment
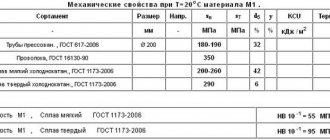
Refining copper phosphate is phosphorus deoxidized hard copper, which is a durable general purpose resin. It is deoxidized by copper phosphorus, in which the residual phosphorus is kept at a low level (0.005-0.013%) to achieve good electrical conductivity. It has good thermal conductivity and excellent welding and soldering properties. The oxide after refining copper in this manner, remaining in the solid copper resin, is removed by phosphorus, which is the most commonly used deoxidant.
The table shows different indicators from annealed (soft) to hard copper state.
| Tensile strength | 220-385 N/mm2 |
| Tensile strength | 60-325 N/mm2 |
| Elongation | 55-4 % |
| Hardness (HV) | 45-155 |
| Electrical conductivity | 90-98 % |
| Thermal conductivity | 350-365 W/cm |
Lead frames connect wiring to electrical terminals on the surface of the semiconductor and large-scale circuitry on electrical devices and printed circuit boards. The material is selected to meet the requirements of the process and to be reliable during installation and operation.
Step-by-step instructions for smelting copper
If you decide to organize a technical process for melting metal at home, first of all, you should know the boiling point of copper. It is 2650°C. At this temperature the metal begins to boil and bubble. A product cast at this temperature will have a high number of pores, which will negatively affect both its mechanical and decorative properties.
If you properly prepare the equipment and organize the melting process, then at home you can obtain high-quality products for both technical, household and decorative use.
Copper wire
To organize the technical process, you will need the following equipment and consumables:
- the form into which the metal will be poured;
- gas-burner;
- bugle;
- charcoal;
- iron wire hook;
- vacuum cleaner with hose;
- iron tongs (for removing the crucible from the furnace);
- crucible for smelting metal (usually ceramic or clay crucibles are used for such purposes);
- muffle furnace.
Brass grades and applications
The brand of brass and its scope of application depend on the composition. For example, tombak, which belongs to the class of wrought brasses, which contains more than 95% copper, can easily combine with steel, forming a bimetal with it. This compound is used in the manufacture of insignia and various objects of art and interior design - figurines, frames, candlesticks.
LO grade brass is used for the manufacture of condenser tubes used in various heating equipment, for example, gas boilers, autoclaves, and bellows.
The LS brand is used to create parts for watch mechanisms, adapters and connecting bushings. Printing matrices are also made from it.
LMC - is found in old Soviet coins with denominations up to 5 kopecks, fittings, nuts and bolts, and its subtype with the prefix “A” is found in parts of river and sea vessels.
Brass, marked LA or LZhM (and its subtypes), is also used for the construction of sea ships and aircraft, various electrical machines and bearings. Very common in parts for various chemical equipment.
Electrolysis of melts
During electrolysis of the melt, the anions of acid residues are oxidized at the anode, and metal cations are reduced at the cathode. There are no water molecules in the system.
For example: electrolysis of molten sodium chloride . Sodium cations are reduced at the cathode
Cathode (–): Na + + ē → Na 0
Chlorine anions are oxidized at the anode :
Anode (+): 2 Cl – – 2ē → Cl2 0
The overall equation for the electrolysis of molten sodium chloride is:
2 Na + Cl – → 2 Na 0 + Cl2 0
Another example: sodium hydroxide electrolysis . at the cathode :
Cathode (–): Na + + ē → Na 0
Hydroxide ions are oxidized at the anode :
Anode (+): 4 OH – – 4ē → O2 0 + 2H2O
The overall equation for the electrolysis of molten sodium hydroxide is:
4 Na + OH – → 4 Na 0 + O2 0 + 2H2O
Many metals are produced industrially by electrolysis of melts.
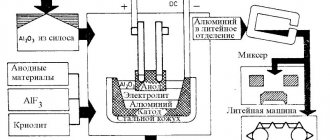
For example , aluminum is produced by electrolysis of a solution of aluminum oxide in molten cryolite. Cryolite – Na3[AlF6] melts at a lower temperature (1100 o C) than aluminum oxide (2050 o C). And aluminum oxide dissolves perfectly in molten cryolite.
In cryolite solution, aluminum oxide dissociates into ions:
at the cathode :
Cathode (–): Al 3+ + 3ē → Al 0
Aluminate ions are oxidized at the anode :
Anode (+): 4Al O 3 3 – – 12ē → 2Al2O3 + 3 O2 0
The general equation for the electrolysis of a solution of aluminum oxide in molten cryolite:
2 Al 2 O 3 = 4 Al 0 + 3 O 2 0
In industry, graphite rods are used as electrodes in the electrolysis of aluminum oxide. In this case, the electrodes are partially oxidized (burnt) in the released oxygen:
C 0 + O2 0 = C +4 O2 -2
Methods Used to Melt Copper at Home
Melting copper at home is possible in several ways. To do this you will need certain tools:
- raw materials;
- heat-resistant crucible;
- fireproof stand;
- wire hook;
- tongs for removing the hot crucible;
- protective equipment: glasses, suit, gloves.
Melting copper at home and in production occurs in the same way. This is achieved by the following methods:
- using a muffle furnace;
- using oxygen flame;
- mountain;
- blowtorch;
- melting in a microwave oven.
The process of melting copper at home
Using a muffle furnace
Casting copper using a muffle furnace is a fairly simple and convenient method. Copper raw materials are crushed into pieces so that they melt faster. The finished material is placed in a graphite crucible and placed in a heated oven. The casting mold must have a higher melting point than the non-ferrous metal.
When the raw material becomes liquid, the crucible is removed from the furnace using tongs. Use a hook to remove the oxide film from the metal surface. Then the liquid is poured into a pre-prepared form.
What does a muffle furnace consist of?
Gas torch or blowtorch
Melting copper with a torch
A special oven can be replaced by a gas burner or a blowtorch. It is placed under the bottom of the container with metal and care is taken that the flame covers the bottom completely.
When using this method, the material quickly oxidizes, therefore, to prevent the formation of a thick oxide film, the raw material is sprinkled with charcoal particles on top.
To melt low-melting alloys made of brass or bronze, a gas torch or blowtorch is sufficient.
Horn
You can melt copper using a forge. To do this, a crucible with crushed raw materials is placed on hot charcoal. To speed up the melting, use a home vacuum cleaner turned on in blowing mode. The pipe must have a metal tapered tip, since the plastic will melt under the influence of high temperature. This method is suitable for those who regularly smelt copper at home.
To increase the temperature, more air must be blown into the forge.
Drawing of a mobile forge
Independent copper smelting
For many people, smelting copper and making all kinds of products from it is an exciting hobby. Those who want to devote their free time to melting metal need to prepare the following devices for work:
- muffle furnace;
- pure raw materials;
- heat-resistant crucible;
- fireproof stand;
- steel wire hook;
- tongs for removing the crucible from the oven;
- personal protective equipment: suit, glasses, gloves.
Actions are performed according to the instructions:
- They put on a special suit.
- The raw materials are crushed and placed in a crucible.
- Place in the oven and set the desired temperature. The metal should not be allowed to boil.
- When the set temperature is reached, open the door, grab the crucible with tongs, remove it from the oven, and place it on a refractory stand.
- Using a steel hook, the oxide film formed as a result of melting is moved to the edges of the container.
- The liquid copper mass is poured into a special container and cooled.
- In powerful muffle furnaces, red copper and all kinds of alloys can be melted.
Melting with a torch
It should be remembered that a nitrogen environment is important during melting. For low-melting copper alloys, brass or some brands of bronze, you can use a regular gas burner. For this you will need:
- feedstock;
- special forms;
- tongs for removing metal from a hot working surface;
- high pressure burner running on gas;
- protective equipment: suit, glasses, gloves.
The alloy melting technology is as follows:
- The raw materials are highly crushed. This can be done using a file, turning the material into sawdust.
- Place in a special form made of heat-resistant material.
- Wear a protective suit, goggles, and thick gloves.
- Light the burner.
- The heating device is directed with free movements along the body of the container. To achieve quick results, the flame should touch the surface with the blue tip. In this place of the torch the temperature is highest.
- After the solid has melted, the crucible is grabbed with tongs.
- The liquid mass is poured into the desired form.
If you don't have a gas burner, you can use an ordinary blowtorch.
When casting non-ferrous alloys, every craftsman must remember safety precautions:
- The room where work is carried out must have good ventilation.
- To avoid burns, it is necessary to work in personal protective equipment.
Optimal air temperature, acceptable air humidity, cleanliness of the workplace, low concentration of harmful atmospheric substances, good illumination of the space are factors that help to avoid injuries.