Types of heat treatment of steel
Annealing
Annealing is a thermal treatment (heat treatment) of a metal that involves heating the metal and then slowly cooling it. This heat treatment (i.e. annealing) comes in different types (the type of annealing depends on the heating temperature and the cooling rate of the metal).
Hardening
Hardening is a heat treatment (heat treatment) of steel and alloys, based on the recrystallization of steel (alloys) when heated to a temperature above critical; After sufficient exposure to the critical temperature to complete the heat treatment, rapid cooling follows. Hardened steel (alloy) has a nonequilibrium structure, so another type of heat treatment is applicable - tempering.
Vacation
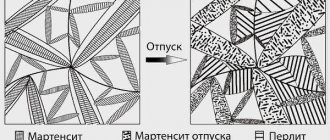
Tempering is a heat treatment (heat treatment) of steel and alloys, carried out after hardening to reduce or relieve residual stresses in steel and alloys, increasing toughness, reducing the hardness and brittleness of the metal.
Normalization
Normalization is a heat treatment (heat treatment) similar to annealing. The differences between these heat treatments (normalization and annealing) are that during normalization the steel is cooled in air (when annealing, it is cooled in a furnace).
Vacation
To normalize the characteristics of steel workpieces after hardening, it is recommended to temper it. Its essence lies in thermal exposure to temperatures at which phase transformation does not occur. The result of this operation will be the homogeneity of the steel structure.
Types of tempering for metal blanks:
- Short. Used for carbon steel grades. The maximum exposure temperature is +200°C. As a result, the fragility index decreases and the tension in the structure decreases. Average. Heat treatment occurs at +400°C. Technology is needed to remove excess carbon. In this case, the crystal lattice becomes cubic. High. Processing temperature – up to +650°С. It is used to achieve optimal characteristics of strength, viscosity and ductility.
The defining indicator for this process is temper brittleness. It indicates the degree to which impact strength drops during sudden temperature changes.
Heating the workpiece
Heating the workpiece is a critical operation. The quality of the product and labor productivity depend on the correctness of its implementation. You need to know that during the heating process the metal changes its structure, properties and characteristics of the surface layer and as a result of the interaction of the metal with atmospheric air, scale is formed on the surface; the thickness of the scale layer depends on the temperature and duration of heating, the chemical composition of the metal. Steels oxidize most intensively when heated above 900°C; when heated to 1000°C, oxidation increases 2 times, and at 1200°C - 5 times.
Chrome-nickel steels are called heat-resistant because they practically do not oxidize.
Alloy steels form a dense, but not thick layer of scale, which protects the metal from further oxidation and does not crack during forging.
When heated, carbon steels lose carbon from a surface layer of 2-4 mm. This threatens the metal with a decrease in the strength and hardness of the steel and hardening deteriorates. Decarburization is especially harmful for small-sized forgings followed by hardening.
Carbon steel blanks with a cross-section of up to 100 mm can be quickly heated and therefore they are placed cold, without preheating, in a furnace where the temperature is 1300°C. To avoid cracks, high-alloy and high-carbon steels must be heated slowly.
When overheated, the metal acquires a coarse-grained structure and its ductility decreases. Therefore, it is necessary to refer to the iron-carbon diagram, which defines the temperatures for the start and end of forging. However, overheating of the workpiece can, if necessary, be corrected by heat treatment, but this requires additional time and energy. Heating the metal to an even higher temperature leads to burnout, which disrupts the bonds between grains and such metal is completely destroyed during forging.
Why should you contact Kubanzheldormash JSC?
JSC Kubanzheldormash has extensive production areas, so we can work with large orders and complete them in the shortest possible time. Also, our employees are highly qualified and have extensive experience in metallurgical work. Our company has been manufacturing railway tools and equipment for decades, so the experience of our employees is not surprising.
We strictly monitor the quality of processed parts, preventing defects. All work is carried out in strict accordance with technological standards, so if for some reason a part does not meet the expected output parameters, we redo everything again. The client and reputation are much more important to us than anything else, so you can rely on heat treatment from Kubanzheldormash JSC.
Heat treatment of metal from JSC Kubanzheldormash is:
- eight decades of experience in heat treatment of various metals;
- highest quality heat treatment;
- the shortest possible time for preparing serial production for specific parts of the Customer;
- flexible pricing policy and competitive prices;
- favorable conditions for regular customers;
- 100% quality control on workshop and laboratory hardness testers Zwick (Germany);
- own chemical laboratory;
- 24/7 work in three shifts;
- international certificate ISO9001;
- delivery to any point in Russia and the countries of the Customs Union.
If you need to place an order for heat treatment of workpieces, parts and assemblies, all you need to do is contact our sales department.
Burnout
Burnout is an irreparable marriage. When forging products from low-carbon steels, less heating is required than when forging a similar product from high-carbon or alloy steel.
When heating metal, it is necessary to monitor the heating temperature, heating time and temperature at the end of heating. As the heating time increases, the scale layer grows, and with intense, rapid heating, cracks may appear. It is known from experience that on charcoal a workpiece 10-20 mm in diameter is heated to forging temperature in 3-4 minutes, and workpieces with a diameter of 40-50 mm are heated for 15-25 minutes, monitoring the color of the heat.
Chemical-thermal treatment
Chemical thermal treatment (CHT) of steel is a set of heat treatment operations involving saturation of the surface of the product with various elements (carbon, nitrogen, aluminum, silicon, chromium, etc.) at high temperatures.
Surface saturation of steel with metals (chromium, aluminum, silicon, etc.), which form substitutional solid solutions with iron, is more energy-intensive and longer lasting than saturation with nitrogen and carbon, which form interstitial solid solutions with iron. In this case, the diffusion of elements proceeds more easily in the alpha-iron lattice than in the more densely packed gamma-iron lattice.
Chemical-thermal treatment increases hardness, wear resistance, cavitation, and corrosion resistance. Chemical-thermal treatment, creating favorable residual compressive stresses on the surface of products, increases reliability and durability.
Hardening defects
Hardening defects include:
- cracks,
- leashes or warping,
- decarbonization.
The main reason for cracks and cracks is an uneven change in the volume of the part when heated and, especially, during sudden cooling. Another reason is the increase in volume during martensite quenching.
Cracks arise because stresses due to uneven changes in volume in individual places of the part exceed the strength of the metal in these places.
The best way to reduce stress is to cool slowly around the martensitic transformation temperature. When designing parts, it is necessary to take into account that the presence of sharp corners and sudden changes in cross-section increases internal stress during hardening.
Warping (or warping) also arises from stress as a result of uneven cooling and manifests itself in the curvature of parts. If these distortions are small, they can be corrected, for example, by grinding. Cracks and warping can be prevented by preliminary annealing of parts, uniform and gradual heating of them, as well as the use of stepwise and isothermal hardening.
Decarburization of steel from the surface is the result of carbon burnout during high and prolonged heating of the part in an oxidizing environment. To prevent decarburization, parts are heated in a reducing or neutral environment (reducing flame, muffle furnaces, heating in liquid media).
The formation of scale on the surface of the product leads to waste of the metal and deformation. This reduces thermal conductivity and, therefore, reduces the rate of heating of the product in the furnace and complicates mechanical processing. Scale is removed either mechanically or chemically (etching).
Carbon burned from the surface of the metal makes the product decarbonized with reduced strength characteristics and difficult machining. The intensity with which oxidation and decarburization occurs depends on the heating temperature, i.e., the greater the heating, the faster the processes occur.
The formation of scale during heating can be avoided if a paste is used for hardening, consisting of liquid glass - 100 g, refractory clay - 75 g, graphite - 25 g, borax - 14 g, carborundum - 30 g, water - 100 g. The paste is applied to the product and allow it to dry, then heat the product in the usual way. After hardening, it is washed in a hot soda solution. To prevent the formation of scale on high-speed steel tools, a borax coating is used. To do this, the instrument heated to 850°C is immersed in a saturated aqueous solution or borax powder.
Coolants
The main coolant for steel is water. If you add a small amount of salts or soap to the water, the cooling rate will change. Therefore, under no circumstances should the quenching tank be used for other purposes (for example, washing hands). To achieve the same hardness on the hardened surface, it is necessary to maintain the coolant temperature at 20 - 30 degrees. You should not change the water in the tank frequently. It is absolutely unacceptable to cool the product in running water.
The disadvantage of water hardening is the formation of cracks and warping. Therefore, only products of simple shapes or cemented ones are hardened using this method.
- When hardening products of complex configurations made of structural steel, a fifty percent solution of caustic soda is used (cold or heated to 50 - 60 degrees). Parts heated in a salt bath and hardened in this solution turn out light. The solution temperature should not be allowed to exceed 60 degrees.
Modes
Vapors formed during quenching in a caustic solution are harmful to humans, so the quenching bath must be equipped with exhaust ventilation.
Be careful not to let water get into the oil bath, as this may cause the product to crack. What is interesting: in oil heated to a temperature above 100 degrees, the ingress of water does not lead to the appearance of cracks in the metal.
The disadvantage of an oil bath is:
- release of harmful gases during hardening;
- formation of plaque on the product;
- oil's tendency to flammability;
- gradual deterioration of hardening ability.
- Steels with stable austenite (for example, X12M) can be cooled with air supplied by a compressor or fan. At the same time, it is important to prevent water from entering the air duct: this can lead to the formation of cracks in the product.
- Step hardening is performed in hot oil, molten alkalis, and low-melting salts.
- Intermittent hardening of steels in two cooling environments is used for processing complex parts made of carbon steels. First they are cooled in water to a temperature of 250 - 200 degrees, and then in oil. The product is kept in water for no more than 1 - 2 seconds for every 5 - 6 mm of thickness. If the exposure time in water is increased, cracks will inevitably appear on the product. Transferring the part from water to oil must be done very quickly.
Do you need to cut metal quickly and efficiently? Use a plasma cutter! How to do it correctly, read this article.
If you are interested in how to turn metal products, read the article at https://elsvarkin.ru/obrabotka-metalla/tokarnaya-obrabotka-metalla-obshhie-svedeniya/ link.
Main Difference – Annealing vs Quenching vs Quenching
Heat treatment is the use of heat to change the properties of a material, especially in metallurgy. It is a type of industrial process that involves changing the chemical and physical properties of metals and metal alloys. There are four main types of heat treatment methods: annealing, tempering, quenching and normalizing. Annealing is a heat treatment process used to soften materials or to obtain other desired properties such as machinability, electrical properties, dimensional stability, etc. Quenching or hardening is the process of increasing the hardness of a metal. Quenching is the process of heating a substance to a temperature below its critical range, holding it, and then cooling it. The main difference between annealing and tempering hardening is that annealing is done to soften the metal or alloy, while quenching is done to increase the hardness of the metal or alloy, and tempering is done to reduce the brittleness of the hardened metal or alloy.
Key areas covered
1. What is Annealing - Definition, Process, Purposes of Annealing 2. What is Tempering - Definition, Process, Types of Tempering Processes 3. What is Tempering - Definition, Process, Austempering 4. What is the Difference Between Curing and Tempering Annealing - Comparison of Key Differences
Key terms: alloy, annealing, casting, carburization, flame hardening, hardening, induction hardening, metal, metallurgy, nitriding, normalization, hardening, surface hardening, tempering
Heat treatment of tool alloys
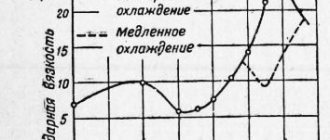
The following statement is true for almost all metals: with increasing tempering temperature, strength decreases and ductility increases.
The only exceptions are high-speed steels used in the production of tools. To ensure better heat resistance and wear resistance characteristics, they are alloyed with carbide-forming elements: molybdenum, cobalt, tungsten and vanadium. And for hardening, heating to temperatures above 1200 °C is used, which allows the formed carbides to be most completely dissolved. The thermal conductivity of iron itself and its alloying elements varies significantly, therefore, to prevent deformation and cracking during heating, temperature pauses should be performed. This occurs when temperatures reach 800 °C and 1050 °C, and for large objects the first interval is assigned at a temperature of 600 °C. The duration of the stop ranges from 5 to 20 minutes, which allows for the best conditions for dissolving carbides. Cooling is most often carried out in oil.
Deformation can be significantly reduced by stepwise heat treatment of steel in molten salts, where hardening is performed at a temperature of about 500 °C. To increase the hardness of the products, this is followed by double tempering at 570 °C. The duration of the process is 1 hour, and its mode is influenced by the chemical properties of alloying elements and temperature, which determines the rate of carbide release.
What is annealing
Annealing is the process of softening a material to obtain the desired chemical and physical properties. Some of these desirable properties include machinability, weldability, dimensional stability, etc. This is a type of heat treatment.
The annealing process involves heating the metal to or near a critical temperature (the critical temperature is the temperature at which the crystalline phase of the metal changes). Heating to such a high temperature makes it suitable for manufacturing. After heating, the metal must be cooled to room temperature. This can be done in the oven.
Figure 1: Annealing a silver strip
Slow cooling of the metal results in a refined microstructure. This may partially or completely separate the components. The annealing process can also be used on pure metals and alloys. According to the process, ferrous metals are classified as below.
- Fully annealed ferrous alloys (use a very slow cooling process)
- Annealed iron alloys process (cooling speed can be faster)
Other metals such as brass, silver, copper can be fully annealed but cool quickly. This can be done by quenching in water.
What is hardening
Hardening is the process of increasing the hardness of a material. Hardening increases the strength of the material. Quenching is often accomplished by quenching . In the metal quenching process, the metal is heated to an austenitic crystalline phase and then rapidly cooled. Cooling can be done either by forced air or other gases such as nitrogen, oil, brine, etc. (selected depending on the type of alloy and its components).
The hardening process increases the strength and wear resistance of the metal. But the presence of sufficient carbon and alloy content is a prerequisite for hardening. Hardening can be done for metal alloys such as steel. However, hardening by this method makes the metal brittle. Therefore, the tempering process is usually followed by a hardening process.
There are two main types of hardening processes; surface hardening and hardening of the body.
Surface hardening
Surface hardening increases the hardness of the outer surface while the core remains soft. Surface hardening can be done by several methods such as carburizing, nitriding and flame hardening/induction hardening.
- In carburization , a metal alloy is placed at high temperature for several hours in a carbonaceous environment.
- Nitriding uses nitrogen and heat. This is typically used for fuel pumps.
- In flame hardening/induction hardening, heat is applied for a short period of time in the form of a flame and the metal is immediately cooled.
Figure 2: Fuel pump
Case hardening
Case hardening increases surface hardness by infusing elements into the surface of the material and forming a thin layer of a harder alloy. Hardening the housing increases the wear resistance of the equipment without changing internal parts.
Classification and types of heat treatment
The fundamental parameters affecting the quality of heat treatment are:
- heating time (speed);
- heating temperature;
- duration of holding at a given temperature;
- cooling time (intensity).
By changing these modes, you can obtain several types of heat treatment.
Types of heat treatment of steel:
- Annealing
- I – kind: homogenization;
- recrystallization;
- isothermal;
- removal of internal and residual stresses;
- full;
- Hardening;
- Vacation:
- short;
- average;
- high.
- Normalization.
Heating temperature of steel during heat treatment
Vacation
Tempering in mechanical engineering is used to reduce the strength of internal stresses that appear during hardening. High hardness makes products brittle, so tempering is used to increase impact strength and reduce the hardness and brittleness of steel.
Vacation low
Low tempering is characterized by the internal structure of martensite, which, without reducing hardness, increases viscosity. Measuring and cutting tools are subjected to this heat treatment. Processing modes:
- Heating to a temperature of 150°C, but not higher than 250°C;
- holding time - one and a half hours;
- cooling - air, oil.
Average holiday
For medium tempering, transformation of martensite into trostite. Hardness decreases to 400 HB. Viscosity increases. Parts that operate under significant elastic loads are subjected to this tempering. Processing modes:
- heating to a temperature of 340°C, but not higher than 500°C;
- cooling - air.
High holiday
With high tempering, sorbitol crystallizes, which eliminates stress in the crystal lattice. Critical parts are manufactured that have strength, ductility, and toughness.
Heating to a temperature of 450°C, but not higher than 650°C.
Annealing
The use of annealing makes it possible to obtain a homogeneous internal structure without stress on the crystal lattice. The process is carried out in the following sequence:
- heating to a temperature slightly above the critical point, depending on the grade of steel;
- holding with constant temperature maintenance;
- slow cooling (usually cooling occurs together with the furnace).
Homogenization
Homogenization, otherwise known as diffusion annealing, restores the non-uniform segregation of castings. Processing modes:
- heating to a temperature from 1000°C, but not higher than 1150°C;
- exposure – 8-15 hours;
- cooling: oven – up to 8 hours, temperature reduction to 800°C;
- air.
Recrystallization
Recrystallization, otherwise low annealing, is used after plastic deformation treatment, which causes hardening by changing the grain shape (hardening). Processing modes:
- heating to a temperature above the crystallization point by 100°C-200°C;
- holding – ½ – 2 hours;
- cooling is slow.
Isothermal annealing
Alloy steels are subjected to isothermal annealing to cause austenite decomposition. Heat treatment modes:
- heating to a temperature of 20°C - 30°C above the point;
- holding;
- cooling: fast - not lower than 630°C;
- slow – at positive temperatures.
Stress Relief Annealing
Removal of internal and residual stresses by annealing is used after welding, casting, and machining. With the application of work loads, parts are subject to destruction. Processing modes:
- heating to a temperature of – 727°C;
- holding - up to 20 hours at a temperature of 600°C - 700°C;
- cooling is slow.
Complete annealing
Full annealing makes it possible to obtain an internal structure with fine grains, which contains ferrite and pearlite. Full annealing is used for cast, forged and stamped workpieces, which will subsequently be processed by cutting and subjected to hardening.
Complete annealing of steel
- heating temperature – 30°C-50°C above point ;
- excerpt;
- cooling to 500°C: carbon steel – temperature reduction per hour no more than 150°C;
- alloy steel – temperature decrease per hour is no more than 50°C.
Partial annealing
With incomplete annealing, lamellar or coarse pearlite is transformed into a ferrite-cementite grain structure, which is necessary for welds produced by electric arc welding, as well as tool steels and steel parts subjected to processing methods whose temperature does not provoke grain growth of the internal structure.
- heating to a temperature above the point or, above 700°C by 40°C - 50°C;
- curing - about 20 hours;
- cooling is slow.
Hardening
Hardening of steels is used for:
- Promotions:
- hardness;
- strength;
- wear resistance;
- elastic limit;
- Reductions:
- plasticity;
- shear modulus;
- compression limit.
What is hardening
Quenching is the process of heating a substance to a temperature below its critical range, holding it, and then cooling it. This is done to obtain the desired properties. Hardening is often carried out on pre-hardened or normalized steel. The tempering process is useful in reducing the brittleness of hardened steel. The temperature to which tempering is carried out directly affects the hardness of the material. Higher temperatures reduce hardness.
Figure 3: Hardened steel colors
Hardening is performed by reheating a metal alloy to a temperature below the critical temperature (the critical temperature is the temperature at which the crystalline phase of the metal changes). The material is then kept at this temperature for some time, followed by cooling. Cooling may be refrigeration or air cooling.
Tempering Subcategory Bainite . Mainly used for ferrous metals such as steel and ductile iron. It is used to improve the mechanical properties of metal alloys by reducing or eliminating distortion.
Difference between curing and tempering annealing
Definition
Annealing: Annealing is the process of softening a material to obtain the desired chemical and physical properties.
Tempering: Tempering or tempering is the process of increasing the hardness of a material.
Quenching: Quenching is the process of heating a substance to a temperature below its critical range, holding it, and then cooling it.
Process
Annealing: The annealing process involves heating the metal to or near a critical temperature, followed by very slow cooling to room temperature in a furnace.
Quenching: In the quenching process, the metal is heated to an austenitic crystalline phase and then rapidly cooled.
Quenching: Quenching is done by reheating a metal alloy to below its critical temperature, holding it for a period of time, and cooling it.
Annealing: Annealing softens materials.
Tempering: Tempering increases the hardness and strength of materials such as metal alloys.
Hardening: Hardening reduces the brittleness of metals.
Applications
Annealing: Annealing is used for metals and metal alloys.
Quenching: Quenching is used for metal alloys with sufficient carbon and alloy content.
Hardening: Hardening is used mainly for steel.
Conclusion
Annealing, curing and tempering are heat treatment processes. The main difference between annealing and tempering hardening is that annealing is done to soften the metal or alloy, while quenching is done to increase the hardness of the metal or alloy, and tempering is done to reduce the brittleness of the hardened metal or alloy.
Link:
1. Himanshu Verma. "Heat Treatment Processes". LinkedIn SlideShare May 4, 2022
Source
| Temperature, °C | Heat colors | Temperature, °C | Heat colors |
| 1600 | Dazzling white-blue | 850 | Light red |
| 1400 | Bright white | 800 | Light cherry |
| 1200 | Yellow-white | 750 | Cherry red |
| 1100 | Light white | 600 | Medium cherry |
| 1000 | Lemon yellow | 550 | Dark cherry |
| 950 | Bright red | 500 | Dark red |
| 900 | Red | 400 | Very dark red (visible in the dark) |
Steel Cementation
Cementation of steel - chemical-thermal treatment of low-carbon steel by surface saturation (from Table 1
A thin film of iron oxides that gives the metal a variety of rapidly changing colors, from light yellow to gray. Such a film appears if a steel product cleaned of scale is heated to 220°C; As the heating time increases or the temperature rises, the oxide film thickens and its color changes. Tarnish colors appear equally on both raw and hardened steel.
With low tempering (heating to a temperature of 200-300°), martensite mainly remains in the steel structure, which, however, changes the lattice. In addition, the separation of iron carbides from the solid solution of carbon in alpha iron begins and their initial accumulation in small groups. This entails a slight decrease in hardness and an increase in the plastic and viscous properties of steel, as well as a decrease in internal stresses in parts.
For low tempering, parts are kept for a certain time, usually in oil or salt baths. If for low tempering parts are heated in air, then tarnish colors appearing on the surface of the part are often used to control the temperature.
| Temperature, °C | Heat colors | Temperature, °C | Heat colors |
| 1600 | Dazzling white-blue | 850 | Light red |
| 1400 | Bright white | 800 | Light cherry |
| 1200 | Yellow-white | 750 | Cherry red |
| 1100 | Light white | 600 | Medium cherry |
| 1000 | Lemon yellow | 550 | Dark cherry |
| 950 | Bright red | 500 | Dark red |
| 900 | Red | 400 | Very dark red (visible in the dark) |
table 1
| Tarnish color | Temperature, °C | A tool to be released |
| Pale yellow | 210 | — |
| Light yellow | 220 | Turning and planing tools for machining cast iron and steel |
| Yellow | 230 | Same |
| Dark yellow | 240 | Coins for coining by casting |
| Brown | 255 | — |
| Brown-red | 265 | Dies, drills, cutters for processing copper, brass, bronze |
| Violet | 285 | Chisels for steel processing |
| Dark blue | 300 | Coining coins for sheet copper, brass and silver |
| Light blue | 325 | — |
| Grey | 330 | — |