Metal extension at home - Machine tools, welding, metalworking
CONTENTS
Application of electrolytic (galvanic) coatings is based on the electrolysis of metals.
When an electric current passes through an electrolyte (a solution of salts, acids and alkalis), positively charged electrolyte ions (cations) and negatively charged ones (anions) are formed in it.
Metal and hydrogen cations move towards the cathode and form a metallic deposit (deposit) on it or are released as a gas. The metal deposit is called electrolytic (plating) coating. Anions move towards the anode and dissolve it if the anode is soluble.
The amount of deposited substance on the cathode, according to Faraday’s law, can be determined by the formula:
G=cIt, where G is the theoretically possible amount of deposited metal, g; c—electrochemical equivalent, g/A*h; I—current strength, A; t is the duration of electrolysis, hours.
Due to the fact that, in addition to the metal, hydrogen is released at the cathode and other processes occur, the amount of actually deposited metal is less than theoretically possible. The ratio of the amount of actually deposited metal to the theoretically possible is called the current output of the metal or efficiency. process (bath).
The thickness of the deposited metal layer is determined by the formula:
b = с*Dk*tn/100y where Dk is the current density, A/dm2; n is the current output of the metal; y is the density of the deposited metal, g/cm3.
Rice. Scheme of electrolytic metal deposition: 1 - bath; 2 - anode rod; 3 — suspension for anode plates; 4 - cathode rod; 5 — suspension for the part; 6 - anode; 7 - part (cathode).
For a given thickness of the metal layer, the duration of the process can be determined using the formula.
Restoring parts using electrolytic coatings has a number of advantages over surfacing: simplicity of equipment; no structural changes occur in the metal of the part; the ability to simultaneously restore several parts. The process makes it possible to restore parts with low wear and produce wear-resistant coatings. The disadvantage of the process is that it is labor intensive, which limits its use when restoring parts with significant wear.
The most widely used are chrome plating and iron plating, less commonly nickel plating, copper plating and galvanizing.
Chrome plating
Electrolytic chromium coatings have high hardness and wear resistance. Therefore, chrome plating restores wear-resistant surfaces with minor wear (piston pairs, distributor spools, piston pins, etc.).
Anodes are made from lead or an alloy of lead and antimony. The ratio of the anode area to the cathode area is taken from 1:1 to 2:1. During the chrome plating process, the anodes are not dissolved. The chromed part is suspended from the cathode.
A solution of chromic anhydride in water with the addition of sulfuric acid is used as an electrolyte. The highest current efficiency is at a ratio of chromic anhydride and sulfuric acid of 100:1. The concentration of chromic anhydride in electrolytes is from 150 to 350 g/l.
Current density - from 15 to 80 A/dm2, voltage - 12-15 V, electrolyte temperature - 40-65°C.
Chrome plating is performed in baths lined with lead, vinyl plastic or other acid-resistant material. The walls of the bathtub are made double. The space between them is filled with water or oil, which is a coolant for heating the electrolyte in the bath.
The design of the bath must include an exhaust hood to remove evaporation products and gases released during electrolysis. Rectifiers VAKG-12/6-300, VAKG-12/600M with a voltage of 12 V, low-voltage generators AND 500/250, etc. are used as DC power sources.
To intensify the electrolysis process, reverse direct current is used (the polarity changes according to a specific program).
The quality of electroplating largely depends on surface preparation and process conditions.
Preparation of parts for galvanic coating includes: cleaning of parts; mechanical processing to give the correct shape to surfaces; preliminary degreasing with solvents; insulation of areas not to be covered with perchlorovinyl tape, PVC-715 enamel, etc.
After this, the part is mounted on hangers and the restoration areas are degreased. Degreasing can be carried out by chemical, electrochemical and ultrasonic methods.
Chemical degreasing is carried out by immersing parts in a hot (60 “C) alkaline solution and holding it in it for 5 to 60 minutes.
Electrochemical degreasing involves immersing parts in an alkaline solution through which a current is passed. The parts serve as the cathode, and the mild steel plates serve as the anode. Degreasing is carried out at a current density of 5-15 A/dm2, an electrolyte temperature of 60-70 °C for 2-3 minutes at the cathode and 1-2 minutes at the anode. After degreasing, wash in water.
To obtain strong adhesion of the coatings to the base metal, it is necessary to activate the surfaces being built up (remove the oxide film). The dissolution of oxides is carried out by chemical or electrochemical etching. Ferrous metals are etched in an aqueous solution of sulfuric or hydrochloric acids. Electrochemical etching of surfaces is carried out in a bath by passing current through the part and the solution.
To obtain high-quality chrome coatings, it is necessary to maintain the relationship between current density and electrolyte temperature.
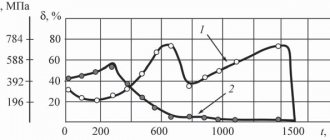
By changing the temperature of the electrolyte and the current density (without changing the composition of the electrolyte), three types of chromium deposits can be obtained: shiny (hardness up to HB 900, high wear resistance and fragility), milky (hardness HB 500-600, sufficient wear resistance and ductility), matte (the hardest and most fragile).
The increased fragility of matte sediment reduces its wear resistance, so this type of sediment is not used when restoring parts. Shiny sediments are used for decorative purposes.
The average current efficiency during chromium plating is 13-15%, and the chromium deposition rate is 0.03-0.06 mm/h.
Due to poor wettability of the surface of the chrome coating, the wear resistance of parts is reduced.
Therefore, when restoring parts operating under conditions of high specific pressure, high temperature and lack of lubrication (piston rings, cylinder liners, etc.)
), porous chrome plating is used. Surface porosity is obtained by mechanical, chemical or electrochemical methods.
With the chemical method, porosity on the coating is obtained by etching in hydrochloric or sulfuric acid.
With the mechanical method, indentations are made on the surface of the part before chrome plating using a cutter, knurling or sandblasting. During the chrome plating process, the prepared surface relief is preserved.
With the electrochemical method, parts are subjected to anodic treatment for 8-12 minutes in an electrolyte of the same composition as during chrome plating.
Ironing
Iron plating is used to restore steel and cast iron parts (bearing seats, holes in connecting rod heads, etc.) with wear reaching 1 mm or more. When restoring parts, iron plating is used more widely than chrome plating.
In contrast to chrome plating, soluble anodes made of low-carbon steel are used in iron plating. Their area should be twice the surface to be coated (cathode). The current efficiency during ironing is 85-95%, the metal deposition rate is 0.2-0.5 mm/h, the hardness of the deposit is HB 700.
The cost of restoring parts using iron is 30-50% of the cost of new parts.
Electrolytes used in ironing are divided into three groups: chloride, sulfate and mixed (sulfate-chloride). The most common are chloride electrolytes, which provide the best quality coatings.
According to the temperature regime, electrolytes are divided into hot (60-90 °C) and cold (18-20 °C).
Hot electrolytes are inconvenient to use, as they require additional heating and temperature control costs, but they provide better coverage.
Of the hot electrolytes, an electrolyte consisting of 200-500 g/l ferric chloride, 100 g/l sodium chloride, acidity (pH) - 08-1.2 is used. Ironing mode: current density - 10-50 A/dm2, temperature 70-80 °C.
Of the cold electrolytes, the most commonly used electrolyte is an electrolyte consisting of 400-600 g/l ferric chloride, 0.5-2.0 g/l ascorbic acid, acidity (pH) - 0.5-1.3. Ironing mode: current density - 10-40 A/dm2, temperature - 20-50 °C.
Rice. 63. Installation diagram for electrolytic deposition of metal
In processes with insoluble anodes (chrome plating), a metal (chromium) is deposited on the cathode, which is the part being built up, resulting from the dissociation of an electrolyte containing chromium salts in the form of chromic anhydride.
In processes with soluble anodes (stagnation), the metal deposited on the part is additionally obtained by dissolving the anode, which is made of this metal. The quality of electrolytic coatings depends on the preparation of the surface of the part, temperature, acidity and composition of the electrolyte, current density at the cathode, the ratio of the cathode and anode areas and a number of other factors.
Restoration of parts by chrome plating. Electrolytic chromium is a silvery-white metal with a bluish tint, characterized by high hardness, low coefficient of friction, high corrosion resistance and wear resistance, is very brittle and is poorly wetted by oil. The melting point of chromium is 1750–1800 °C. Electrolytic chromium adheres well to steel, nickel, copper and its alloys.
According to their intended purpose, chrome coatings are divided into wear-resistant (hard) and protective and decorative. Wear-resistant coatings are used to repair worn surfaces of parts, as well as to increase the wear resistance of parts in order to increase their service life. Wear-resistant chrome coatings can be of two types: smooth and porous. The latter are applied to the surface of parts operating under conditions of high loads and boundary friction.
Protective and decorative coatings are used to protect parts from corrosion and give them a beautiful appearance. The thickness of the protective and decorative coating layer is 1-2 microns, the wear-resistant coating is 0.4-0.5 mm. If the thickness is greater, the chrome coating is of poor quality.
Chromium plating repairs worn-out shaft journals, axles, engine valve and pusher rods, piston pins, precision fuel equipment pairs and other parts.
The parts restoration process can be divided into three stages: preparing parts for coating, applying the coating, and processing the parts after coating.
The technological process of restoring parts by chrome plating consists of a number of operations that must be performed in the following sequence: 1. Mechanical processing. The surfaces of parts to be chrome-plated should be ground until signs of wear are removed and polished. After machining, they should not have holes, cracks or deep marks, since the chrome coating reproduces these defects. Cylindrical grinding machines are used for grinding and polishing. 2. Washing. Parts can be washed in kerosene, white spirit or dichloroethane, or by boiling in a 10% caustic soda solution. Washing is carried out in special baths and then blown with compressed air. 3. Control. Dimensional control is carried out to determine the required thickness of the chromium layer and the chromium plating time, taking into account the allowance for subsequent machining. 4. Insulation of areas not subject to chrome plating. This work is carried out on assembly tables (workbenches), using perchlorovinyl varnish, AK-20 varnish, vinyl plastic or vinyl chloride insulating tape for insulation. Holes that are not subject to chrome plating are closed with lead plugs or other acid-resistant materials. 5. Installation of parts on the suspension. The parts are mounted on a special suspension. In this case, it is necessary to ensure that there is reliable contact between the parts and the current supply rods. Work is carried out on assembly tables. 6. Degreasing. Electrolytic degreasing is carried out in a solution of the following composition: caustic soda—10 g/l; liquid glass - 3 g/l; soda ash - 25 g/l; trisodium phosphate - 25 g/l. The ratio of the anode area to the cathode area is 4:1. The solution temperature is 70–80 °C and the current density is from 5 to 10 A/dm2. Voltage 6-8 V. Process duration 1-2 minutes. Degreasing is carried out in special baths. 7. Washing. Together with the suspension, the parts are washed in running hot water (60-80 ° C), and then in running cold water. Washing is carried out in specially equipped baths. 8. Picking. Electrochemical pickling is carried out in a chrome plating bath or in a bath with a chrome electrolyte. Picking up of parts is carried out for 30-90 s at a current density of 25-40 A/dm2, and for cast iron parts - for 25-30 s at a current density of 20-25 A/dm2. The electrolyte temperature in all cases should be 55-60 °C. Anodic pickling is carried out to remove oxide films from the surface of the part and reveal its structure. After pickling, the parts are washed in distilled water. 9. Chrome plating. Pendants with parts are placed in a chrome plating bath, heated with the current turned on for 5-6 minutes, and then full current is given according to the chrome plating mode. When chrome plating, insoluble anodes made of lead or a lead alloy with 6% antimony are used. The cathode is the part being restored. For chrome plating, electrolytes are most often used, consisting of an aqueous solution of two components - chromic anhydride CrO3 and sulfuric acid H2S04.
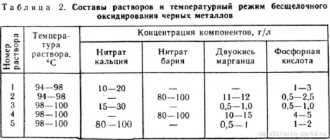
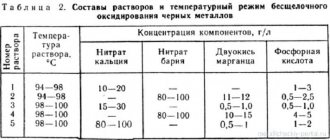
The composition of electrolytes and the bath mode are given in table. 7.
Conventionally, coatings are divided into the following types: milky, shiny and matte. Milk coatings have high wear resistance and increased viscosity; they are recommended to be applied to the surfaces of parts operating under high pressures and alternating loads. Shiny coatings are characterized by high hardness, increased wear resistance, porosity and fragility. However, such coatings have poor wetting ability with respect to oil, and if there is insufficient lubrication, seizures are possible. In these cases, the shiny coating is subjected to anodic etching, i.e., a plus is connected to the part, and a minus is connected to the lead plates.
In this case, porous chrome plating is obtained, which works well under conditions of friction with lubricant. They are applied to the surfaces of wear parts. Both types of coatings can be applied to the surfaces of parts operating under stationary conditions.
Matte coatings are characterized by high hardness and brittleness, as well as low wear resistance. They are used as protective and decorative. Electrolytes for chrome plating (see Table 7) have a number of disadvantages: low yield of chromium flow (12-16%); the need for frequent adjustments due to the instability of the composition; low productivity (a layer 0.1-0.03 mm thick is deposited in 1 hour).
The principle of self-regulation of the electrolyte is that ions are introduced into the electrolyte not in the form of sulfuric acid, but in the form of salts of sparingly soluble strontium sulfate, which is taken in excess, so that part of it is in solution in the form of dissociated ions, and part of it is in the solid. condition at the bottom of the bath.
Table 7
With this electrolyte composition, the required concentration of components is automatically maintained constant, since excess salts located at the bottom of the bath will dissolve in the electrolyte (within the limits of possible solubility). When the concentration of chromic anhydride decreases, the opposite phenomenon will occur—some of the dissolved salts will precipitate at the bottom of the bath in the form of a solid precipitate, thus ensuring a constant concentration of the electrolyte components.
The material for the anodes is POS40 tin-lead solder. Self-regulating electrolyte has the following advantages: productivity is 2 times greater than conventional electrolyte; high current yield of chromium (18-22%); electrolyte stability, resulting in no need for frequent adjustments.
In practice, direct current of constant polarity and reversible direct current (the polarity changes according to a certain program) are used to power galvanic baths. Low-voltage generators AND-500/250, AND-1000/500, AND-1500/750 are used as DC power sources (in the numerator - current at a voltage of 6 V, in the denominator - at a voltage of 12 V), selenium rectifiers of the VSMR type , silicon rectifiers such as VAKG, etc. Parts are chrome-plated in specially designed baths. The bath body is a welded rectangular tank made of sheet steel 4-6 mm thick. The body is inserted into another steel welded tank, which is a casing. The space between the housing and the casing is filled with water, which serves to uniformly heat the electrolyte and maintain its temperature within specified limits. The water in the casing is heated with steam or electricity. To protect the inner surface of the walls of the bathtub body from the aggressive effects of the electrolyte, it is lined (lined) with a material that is chemically resistant to electrolytes: lead, vinyl plastic, asbestos vinyl, acid-resistant tiles. 10. Washing. Pendants with parts are washed in a bath of distilled water to collect chromic anhydride remaining on the parts. 11. Washing. Pendants with parts are washed in baths with running cold and hot water. 12. Dismantling of parts. The parts are removed from the suspension and the insulation is removed from them. This work is carried out on the assembly table. 13. Drying of parts is carried out for 2-3 hours at a temperature of 150-200 ° C in a special drying cabinet. 14. Sanding. When grinding chrome, soft or medium hard wheels should be used. Grinding is carried out with intensive liquid cooling and at a wheel rotation speed of 20-30 m/s and higher. The rotation speed of the part should be 12-20 rpm. Grinding is carried out on cylindrical or internal grinding machines, depending on the configuration of the part. 15. Control. When inspecting parts, the quality of coatings is checked by external inspection and measuring the hardness of the coating. During an external inspection, it is necessary to pay attention to gloss, peeling and density of sediment, spotting, uniformity, lack of peeling and other visible defects. Chromium plating of parts is a very expensive technological process due to its duration and relative complexity, so the technical and economic efficiency will be high only with a large production program (especially small parts). Large basic parts (cylinder blocks, gearbox housings) and parts with complex configurations (crankshafts and camshafts) can be restored by applying a coating using a bathless method. The principle of bathless chrome plating is that a local bath is created in the coating area. Bathless electrolytic deposition of metal can occur in several ways, including jet and flow.
This process can be successfully applied in repair plants and central repair shops, as it requires sophisticated equipment and large production areas.
One of the new progressive methods of coating parts, providing increased process productivity and reduced labor intensity, is chrome plating using a current of alternating polarity, i.e. reverse chrome plating. The composition of the electrolyte is as follows: chromic anhydride CrO3 - 250 g/l, sulfuric acid H2S04 - 2.5 g/l. Current density is 120–130 A/dm2, electrolyte temperature is 52 °C. The duration of the cathodic period is 1-5 minutes, the anodic period is 1-5 s. Current of alternating polarity is obtained by periodically changing direct current using automatic machines ART-1, ART-2, etc. With reverse chrome plating, productivity increases by 2.5-3 times, wear resistance of the coating increases by 30-60%, the coating layer can be obtained up to 1 mm .
Restoration of parts by remaining. By electrolytically growing steel (staying), it is possible to obtain a coating with a hardness of HRC 50-56 without subsequent heat treatment, which is characterized by fairly good wear resistance. The coating thickness can be obtained up to 3 mm. Smooth coatings with microhardness up to 3000 MPa (300 kgf/mm2) can be obtained with a thickness of up to 3 mm or more; coatings of higher hardness (up to €500 MPa) can be obtained with a thickness of 0.8–1.2 mm. The adhesion strength of the coating to the base metal is quite high, resulting in reliable operation of the repaired part under alternating loads.
The technological process of cooling has much in common with the technological process of chrome plating. It also consists of three stages: preparing parts for coating; coating and processing of parts after coating.
The scheme of the technological process of cooling is as follows: mechanical treatment of surfaces; washing with gasoline; installation of parts on the suspension; insulating areas of parts that are not to be coated, degreasing parts with Vienna lime; rinsing with cold running water; anodic treatment in a 30% sulfuric acid solution, rinsing with cold water, rinsing with hot water (at a temperature of 50-60 °C), coating; washing with hot water (temperature 80-90 °C), neutralization with a 10% caustic soda solution; washing with hot water (80-90 °C); dismantling parts from the suspension and removing insulation; mechanical treatment of the coating surface and quality control. Many cooling operations are the same as chrome plating operations, or similar to them, so below we will consider only those operations that differ in content from chrome plating operations.
Insulation of areas of parts not to be coated. Tsapon varnish, enamel, BF-2 glue, bakelite varnish, rubber, vinyl chloride compound and enamels are used as insulating materials.
Anodic treatment is carried out in a bath with an electrolyte of the following composition: 30% aqueous solution of sulfuric acid and ferrous sulfate (ferrous sulfate FeSCv7H20) in an amount of 10-25 g/l of water. The electrolyte density is 1.23 g/cm3. The anode is the workpiece, the cathode is lead or stainless steel plates. The area of the cathodes should be 3-4 times larger than the area of the anodes.
Treatment mode: current density—10–70 A/dm2, electrolyte temperature—16–22 °C, treatment duration—0.5–4 minutes.
Coating. To remove the passive film formed during anodic treatment, the suspension with parts is immersed in a cooling bath and kept in it without current for 10-50 s. Then turn on a current with a density of 5 A/dm2 and within 5-10 minutes bring the current density to the specified value.
Existing hot chloride electrolytes for cooling differ both in composition and in the concentration of their components. They allow you to create coatings of varying hardness. To obtain hard wear-resistant coatings in practice, an electrolyte of the following composition (g/l of water) is successfully used: ferric chloride FeCl2'4H20 - 200-220, hydrochloric acid HC1 - 0.8-1.0. Bath operating mode: current density - 40-50 A/dm2, electrolyte temperature - 75-80 °C. The holding time of parts in the cooling bath depends on the required thickness of the coating layer. The rate of metal deposition onto the part is 0.3–0.5 mm/h.
When remaining, soluble anodes made of low-carbon steel grades 10, 15 or 20 are used. The dissolution of anodes during the electrolysis process causes contamination of the electrolyte with anode sludge (insoluble particles), which in the form of inclusions enters the galvanic coating, deteriorating its quality. The cooling installation must have a device for filtering the electrolyte.
The high temperature of the cooling process (60–80 °C) promotes evaporation of the electrolyte, so the installation must also have a device for replenishing the electrolyte with water and hydrochloric acid.
Hot chloride electrolytes are highly aggressive towards most metals and their alloys. Cooling baths, settling tanks, dosing tanks, pickling baths and other equipment must be protected from the aggressive action of the electrolyte by carbon-graphite antigmite tiles. acid-resistant enamel, acid-resistant rubber, hard rubber or acid-resistant varnishes.
Restoration is used to restore valve stems, pushers, drive rollers (oil, water and other pumps), shafts and axles: transmissions, pulleys, brackets, hubs and other parts of road vehicles.
To restore the seats of body parts in the repair industry, local (outside) melting is used. By leaving it outside the bath, you can restore bearing housings, for example, gearbox housings (Fig. 64). The crankcase is washed in a 10% solution of sodium hydroxide and hot water. The surfaces of the nests are cleaned with emery cloth, washed with gasoline and hot water. Then degrease with Vienna lime and wash with hot and cold water. A device for cooling is installed in the box body. Pour electrolyte into the bath, heated to 40-50 °C. Electrolyte composition: 500 g/l iron dichloride and 1.0-1.5 g/l hydrochloric acid. Then a cylindrical anode 3 made of steel grades St.2, St. is installed in the center of the bath. 3 of such a diameter that the distance between the surfaces of the anode and the hole is at least 40-50 mm, connect the negative pole of the current source to it, and the positive pole to the box body. Turn on the current to pick up the surface of the hole for 4-5 minutes with a density of 10-15 A/dm2, then switch the poles: minus to the part, and plus to the anode, and leave the surface at the same current density for the time required to apply the coating layer of the desired thickness. The layer deposition rate is 0.10–0.15 mm/h. After cooling is complete, the electrolyte is sucked out of the bath with a rubber bulb, the surface is thoroughly washed with hot water, and the device is removed. Wipe the surface of the hole with a swab moistened with a 10% solution of caustic soda; to neutralize residual acid, it is washed with cold water and wiped with a dry rag. Then the hole is bored to the required size.
Coating quality control is carried out by external inspection using a magnifying glass and the surface quality is compared with the standard. If necessary, the hardness of the coating is checked. If the coating peels off, has cracks, holes, stripes, then it is rejected.
Rice. 64. Scheme of local retaining of the gearbox housing bearing housings: 1 - gearbox housing; 2 - electrolyte; 3 - anode; 4 — rubber gasket; 5 — glass; 6 — sliding stop; 7 — support plate; 8 - covers; 9 - ring; 10 - rectifier
The advantages of the cooling process include: high hardness and wear resistance of the coating; high adhesion strength of the coating to the base metal, ensuring reliable operation of parts under difficult operating conditions; obtaining a coating up to 3 mm thick; high current efficiency - 6-7 times more than with chrome plating; lower cost of the process compared to chrome plating; the cost of parts repaired using this method is 2-4 times lower than the cost of new ones.
The disadvantages of cooling include some difficulty in preparing parts for coating and the need for frequent filtration and adjustment of the electrolyte. It should be noted that the use of solid cooling at repair plants provides a great economic effect. Organization of workplaces. Grinding workplaces are located in machine shops or departments. Workstations for galvanic extension of metal are located in specially designated rooms, which are called galvanic shops or areas.
The equipment is installed in the order of technological operations. A technological map should hang above each workplace. Baths for degreasing, chrome plating, cooling, if possible, are located near walls with windows. Wooden gratings covered with corrugated rubber mats should be laid on the floor near workplaces. The distance between the sides of the baths should be 100-150 mm, so that when carrying pendants with parts from one bath to another, solutions of acids and alkalis do not get on the floor and clothes of workers. The site must have wide passages for transporting parts and free access to equipment. Workplaces for degreasing, chrome plating, and cooling baths must be equipped with supply and exhaust ventilation. A monorail with an electric hoist must pass over the work stations for lifting and lowering heavy baskets and hangers with parts.
Metal extension at home
Copper plating is the process of applying a copper layer to the surface using an electroplating method.
The copper layer gives the product visual appeal, which allows the use of copper electroplating in design projects. It also gives the metal high electrical conductivity, which allows the product to be subjected to further surface treatment.
Copper plating can be used as the main process to create a surface layer, and also as an intermediate operation for the subsequent application of another metal layer. This method includes, for example, the process of silvering, chrome plating or nickel plating.
Copper plating can be done at home. This makes it possible to solve many everyday problems.
Electroplating at home: equipment and materials
To perform copper coating yourself, you need to purchase the necessary equipment and materials for the process.
First of all, you need to prepare a source of electric current. Various home craftsmen advise using current strength, which varies over a wide range. Work must be carried out on direct current.
As a current source, you can take a KBS-L battery with a voltage of 4.5 volts or a new Krona brand battery with an operating voltage of 9 volts. You can also use a low-power rectifier instead, which produces a voltage of no more than 12 volts, or a car battery.
It is mandatory to use a rheostat to regulate the voltage and smooth exit from the process.
For the electrolyte solution, a neutral container should be prepared, for example, made of glass, as well as wide plastic dishes that are sufficiently sized to accommodate the part. Containers must withstand temperatures of at least 80°C.
You will also need anodes to ensure coverage of the entire surface of the part. They are designed to supply current to the electrolyte solution and distribute it over the entire area of the part.
To carry out electroplating at home, you will also need chemicals to prepare the solution:
- copper sulfate,
- hydrochloric or other acid,
- distilled water.
Having prepared everything you need, you can start working.
:
Copper plating of steel products
Copper plating of steel with copper sulfate is one of the main processes in the field of electroplating because it is used to pre-plate copper. It has high adhesion to the steel surface, unlike other metals that do not have good adhesion to steel. If the technology is followed, the copper layer adheres perfectly to steel products.
There are two coating technologies: with immersing the product in an electrolyte solution and a method of non-contact coating of the surface with copper without placing it in a liquid electrolyte solution.
Copper plating by immersion
The process is carried out following the following steps:
- The oxide film is removed from the surface of the steel part using sandpaper and a brush, and then the part is washed and degreased with soda and a final rinse with water.
- Two copper plates are placed in a glass jar, connected to copper conductors, which serve as the anode. To do this, they are connected together and connected to the positive terminal of the device used as a current source.
- The workpiece is suspended freely between the plates. The negative pole of the terminal is connected to it.
- A tester with a rheostat is built into the circuit to regulate the current.
- An electrolyte solution is prepared, which usually includes copper sulfate - 20 grams, acid (hydrochloric or sulfuric) - from 2 to 3 ml, dissolved in 100 ml (preferably distilled) water.
- The prepared solution is poured into a prepared glass jar. It should completely cover the electrodes placed in the jar.
- The electrodes are connected to a current source. Using a rheostat, the current is set (10-15 mA should be per 1 cm2 of part area).
- After 20-30 minutes, the current is turned off, and the copper-plated part is removed from the container.
:
Copper plating without immersion in electrolyte solution
This method is used not only for steel products, but also for aluminum and zinc products. The process goes like this:
- A stranded copper wire is taken, the insulating coating is removed from one end, and the copper wires are given the appearance of a kind of brush. For convenient use, the “brush” is attached to a handle-holder (you can take a wooden stick).
- The other end of the wire without a brush is connected to the positive terminal of the voltage source being used.
- An electrolyte solution is prepared based on concentrated copper sulfate with the addition of a small amount of acid. It is poured into a wide container, necessary for convenient dipping of the brush.
- The prepared metal part, cleaned of the oxide film and degreased, is placed in an empty bath and connected to the negative terminal.
- The brush is moistened with the prepared solution and moved along the surface of the plate without touching it.
- Once the required copper layer has been achieved, the process ends and the part is washed and dried.
There should always be a layer of electrolyte solution between the surface of the part and the improvised copper brush, so the brush must be dipped in the electrolyte constantly.
Copper plating of aluminum with copper sulfate
Copper coating is a great way to update aluminum cutlery and other aluminum products used in the home.
Copper plating of aluminum with copper sulfate can be done independently. A simplified option to demonstrate the process is to coat a simple shaped aluminum plate with copper.
You can practice with this example. The process goes like this:
1. The surface of the record must first be cleaned and then degreased.
2. Then you need to apply a little concentrated solution of copper sulfate (copper sulfate) to it.
3. The next step is to connect the wire connected to the negative pole to the aluminum plate. You can connect the wire to the plate using a regular clamp.
4. A positive charge is applied to a device consisting of bare copper wire with a diameter of 1 to 1.5 mm, the end of which is distributed between the bristles of the toothbrush.
During operation, this end of the wire should not touch the surface of the aluminum plate.
5. Having dipped the bristles in a solution of copper sulfate, begin to move the brush in the place prepared for coating with copper. In this case, there is no need to close the circuit by touching the surface of the aluminum plate with the end of the copper wire.
6. Copper plating of the surface immediately becomes visually noticeable. In order for the layer to be of high quality, there is no need to rush to complete the process.
7. After completing the work, the copper layer must be leveled by additional cleaning, removing the remaining copper sulfate and wiping the surface with alcohol.
Galvanoplasty at home
Galvanoplasty is the process of electrochemical action on a product in order to give it the required shape by depositing metal on the surface.
Typically this technology is used for metal coating of non-metallic products. It is widely used in jewelry and the design of household items.
The coating of the work product must have electrically conductive properties. In the absence of such a layer, the object is first coated with graphite or bronze.
Electroplating at home is especially popular among craftsmen. To create the desired shape, a cast is made from the copy. For this purpose, easily melting metal, graphite and gypsum are used.
:
After the mold is made, the object is plated using an electrolyte.
Ironing and more. Part 1.
The ironing process is the electrolytic precipitation of iron from electrolyte solutions of its salts.
Iron is deposited on the cathode, and strips of low-carbon steel are used as the anode. The iron plating process produces a coating of chemically higher purity, making it more resistant to corrosion than mild steel. The ironing process is used to build up metal on the damaged surface of steel and cast iron parts to restore their parameters in various fields of industry:
- In printing, iron plates are used to make clichés using the electroplating method, and also protect copper plates from oxidation by printing ink.
- In the automotive industry, with the help of iron, the dimensions of worn-out machine parts are restored using the method of galvanoplasty.
- In mechanical engineering, machine parts are restored by ironing.
- In electrical engineering, parts of power tools are restored using iron ironing.
Restoration of parts using iron plating is carried out using the galvanoplasty method. (See "What is electroforming? Part 1, Part 2").
The ironing process is very effective , since the electrolyte components are inexpensive, the growth rate is quite high, and the coating can be obtained up to 8 mm thick.
To obtain wear-resistant coatings with increased mechanical, magnetic properties and improved structure, the ironing process is carried out in ironing electrolytes containing various additives, for example, nickel, manganese, chromium (will be discussed in the article “Iron ironing and more. Part 2”).
The ironing process can be carried out from solutions of sulfuric acid or chloride ferrous salts. Sulfuric acid electrolytes are less aggressive, but lower in performance, and the deposits are more fragile and stressed.
In repair practice, the most widely used are iron chloride electrolytes, which provide dense, fine-grained sediments up to 3–5 mm thick with high mechanical properties and a deposition rate of 0.4–0.5 μm/h.
Four types of iron chloride electrolytes, differing in the concentration of iron salts, are widely used: low concentration (200 - 320 g/l) (type I), medium (400 - 450 g/l) (type II), high (600 - 680 g/l) l) (type III) and optimal (300 – 350 g/l) (type IV).
The first iron electrolyte is used to restore parts that require a hard iron coating. At a temperature of 60 – 80 0 C and DC = 30 – 50 A/dm 2, dense coatings up to 1.5 mm thick are obtained.
The second iron electrolyte is intended for the restoration of parts with low hardness. It provides high-quality coatings up to 2 mm and hardness HV = 250 – 450.
III-iron electrolyte at a temperature (75 – 95 0 C) and low current density makes it possible to obtain soft and viscous coatings up to 3 mm thick.
The fourth iron electrolyte has significant advantages: the anodic current output is equal to the cathode, therefore the concentration of iron in the iron electrolyte remains constant, and the coating is wear-resistant.
Composition of the most universal iron electrolyte, g/l:
Iron chloride (FeCl2∙4H2O) 300 – 330
Hydrochloric acid (HCl) 1.5 ml – 2 ml
Temperature 75 – 80 0 C, cathode current density 4 – 5 A/dm 2 to 10 – 20 A/dm 2.
The ratio of the anodic surface to the cathodic surface is 2: 1
Steel strips placed in fiberglass covers are used as anodes.
The hanging of parts into the iron-plating electrolyte is carried out without current, while the parts heat up and the passive film on them is destroyed. After 10 – 30 seconds, the current is set to 4 – 5 A/dm 2 and in 10 minutes its value is increased to 10 – 20 A/dm 2. After ironing, the parts must be washed in hot water, neutralized in a 5–10% soda solution and rinsed again in water.
The pH of the ironing electrolyte is adjusted taking into account the consumption of HCl per 1 A/h of 0.8 g of acid.
Thus, by organizing an ironing section, you can make a good profit from restoring complex, expensive machine parts and various mechanisms.
Metal extension at home - Metalist's Handbook
The main task of electroplating with copper at home, or copper plating in other words, is to prepare the metal surface for further processing. Various metals and non-metals can be subjected to this operation, among which are:
- steel,
- brass,
- nickel and others.
Use of copper
Due to its numerous advantages, this metal has become widespread. Today, copper and its numerous alloys are widely used in industry.
The metal is relevant for aircraft manufacturing, automotive manufacturing, instrument making and other industries. Metal and products made from it are no less popular in the domestic sphere.
Copper plating itself is one of the best ways to coat a metal surface with a thin layer. At home, copper plating can be done in several ways.
Galvanic copper plating at home
For this you will need:
- Copper sulfate;
- Water;
- Hydrochloric acid in its pure form.
Galvanic copper plating at home
Preparation of the solution
Copper sulfate
We make a saturated solution of copper sulfate, after which you will need to add 1/3 of this solution to hydrochloric acid. After preparing the copper sulfate solution, it should be thoroughly stirred so that there are no particles.
Next, you need to add hydrochloric acid in a thin stream to this solution. Don't forget about safety precautions and use gloves and safety glasses.
After you have added hydrochloric acid to the solution, it should be mixed thoroughly.
So, the solution is ready and you can start copper plating at home. To do this, you need to take the metal part on which you are going to apply a layer of copper and prepare it for work. Preparation includes sanding it with sandpaper.
This procedure allows you to not only clean the metal surface, but also degrease it. The same procedure will be relevant for parts made of brass or lead. After this, the coating must be thoroughly washed in a solution of soda ash.
This will allow the material to be degreased more thoroughly.
Soda ash for degreasing material
Next, the surface must be immersed in a solution of copper sulfate and hydrochloric acid. Please note that the first layer of copper is very thin and weak, so it is advisable to remove it with a wire brush.
After you have done this, the surface of the steel or lead should be washed again in a solution of soda ash and again immersed in the copper plating solution.
These manipulations will lead to the fact that the layer of copper on the surface at home will be much thicker and much stronger, since it can be removed from the object only using sandpaper, and not a metal brush as before.
This method allows you to make a very high-quality copper coating that can only be removed with sandpaper. To improve the copper coating at home, the part should be immersed in the solution again. This method is distinguished by its simplicity and high efficiency, including for lead products.
Copper plating procedure
Copper plating is usually called the procedure for galvanic deposition of copper; the thickness of the copper layer in such cases can be from 300 microns or more. Copper plating of steel is one of the most important processes in electroplating, as it is used as an additional process before applying other metals for chrome plating, nickel plating, and silver plating.
The copper layer adheres perfectly to the steel and is able to smooth out various defects on the surface.
Copper coatings are characterized by high adhesion to other surfaces, lead products, especially metal ones, as well as high electrical conductivity and ductility. The newly applied coating has a bright pink matte or shiny color. Under the influence of atmospheric influences, copper coatings can oxidize and become covered with a coating of oxides with various rainbow-colored spots.
Areas of use of copper plating
Generally, electroplating copper plating can be used:
- For decorative purposes. Given the enormous popularity of antique copper products these days. There are methods of artificial aging of steel products;
- In galvanoplasty. Widely used in jewelry, among souvenirs, for making bas-reliefs, etc.;
- In the technical industry. Copper plating of metal is very important in the electrical field. The low cost of copper plating compared to gold or silver coatings makes it possible to reduce the cost of manufacturing electrodes, electrical busbars, contacts and other elements from lead steel.
Copper plating occurs together with the application of other galvanic coatings
- If you need to apply a multi-layer protective and decorative coating to a layer of steel. In the vast majority of cases, copper is used here together with nickel and chromium. This allows you to improve adhesion to the base metal and obtain a shiny, high-strength coating;
- To avoid cementation of the area. Copper plating of lead will prevent carbonization from occurring on steel areas. To apply the copper layer, use only those areas where cutting will be carried out;
- When performing restoration and restoration work. This method is most often used to restore chrome parts of cars and motorcycles. For these purposes, a fairly thick layer of copper is applied, about 100-250 microns or more, which makes it possible to cover all defects and damage to the metal for applying subsequent coatings;
Types of copper plating
- Using electrolyte immersion;
- Without immersion in electrolyte.
The first method involves treating a metal product with sandpaper, a brush and rinsing with water.
After which degreasing in a hot soda solution with repeated rinsing. Next, two copper plates – anodes – are lowered into a glass container on copper wires.
The part is suspended on a wire between the plates, after which the current is started.
The second method is relevant for products made of steel, aluminum and zinc.
Home copper plating
This procedure is relevant for various cases, since applying a layer of copper can be used for aluminum cutlery, souvenirs, candlesticks, etc.
Non-metal products on which a layer of copper has been applied have a unique effect. These can be plant stems, leaves, etc.
Due to the fact that there is no conductive layer in the objects being coated, a special electrically conductive varnish is used instead, which is applied to the surface.
The varnish contains a number of organic solvents, foaming agents and finely dispersed graphite powder, which creates electrical conductivity.
The varnish is applied in a thin layer to a dry surface, and after drying in an hour, you can begin copper plating. If desired, copper can be given different color shades using special methods.
The high quality and uniqueness of such products is deservedly equated to real jewelry.
: Copper plating at home
Do-it-yourself electroplating at home: technology and equipment
Galvanics is also a branch of applied science “Electrochemistry”, which studies the processes that occur during the deposition of metal cations on a cathode placed in an electrolytic solution, and the technological process. Electroplating at home or performed in production allows you to apply a thin layer of metal to the surface of the workpiece, which can act as a protective or decorative coating.
Home electroplating installation
Methods for implementing such a technological process, which is quite complex, have already been well developed, so today it is actively used not only by manufacturing enterprises, but also by many home craftsmen.
Process Features
The coating formed on the workpiece using galvanization can be applied for technological purposes or perform decorative, protective, or both functions at once. For decorative purposes, a thin layer of gold or silver is created, and to ensure reliable protection of the surface of the workpiece from corrosion, galvanizing or galvanic copper plating is performed.
Electrolysis process diagram
It is not difficult to do electroplating even at home. This procedure is performed as follows.
- Two anodes are lowered into a dielectric container with electrolyte, connected to the positive contact of the electric current source. The material used to manufacture such anodes must be metal, the layer from which must be formed.
- The workpiece itself, connected to the negative terminal of the electric current source and thus acting as a cathode, is placed in the electrolyte between the anodes.
- Galvanization, that is, the process of transferring metal molecules from the electrolyte to the cathode product, begins to occur at the moment when the resulting electrical network is closed.
Application of technology
Electroplating is often used in relation to various elegant objects (jewelry, orders and medals, coins, shells, flower pots, sculptures, portraits, etc.). Copper is most often used in electroforming. However, other metals can also be used, including nickel, chromium, steel, and silver.
If all technological requirements are met, it is possible to distinguish a copied object from an original one only by the barrier layer or by removing the original. Moreover, it is quite possible to do all the work yourself at home.
Note! The coating of the copied product must be electrically conductive. If the material lacks this property, bronze or graphite is applied to it.
Creating a Form
We take a print from the product that we will copy. To do this you will need some kind of low-melting metal, plasticine, plaster or wax. If we use metal, we treat the item being copied with soap and put it in a cardboard box. Next, pour in a low-melting alloy.
When the casting is completed, we take out the product and subject the resulting form to first degreasing and then copper plating in an electrolyte. To avoid metal deposits on those sides where there is no imprint, we melt the metal in boiling water to obtain a matrix. Fill the form with plaster. The output is a copy.
To create a matrix you will need the following composition:
- wax - 20 parts;
- paraffin - 3 parts;
- graphite - 1 part.
If the mold is created from a dielectric material, we apply an electrically conductive coating to its surface. The conductor layer is applied either by metal reduction or mechanically, which involves applying flake graphite using a brush.
Even before mechanical surface treatment begins, we grind the graphite in a mortar and sift it through a sieve. The best adhesion of graphite is observed with plasticine.
It is most effective to treat plaster, wood, glass and plastic forms, as well as papier-mâché, with a solution of gasoline and wax.
When the surface has not yet dried, we apply graphite dust to it, and blow off the adhering substance with a directed air flow.
The electroplated coating is easy to separate from the matrix. If the mold is metallic, we create an oxide or sulfide conductive film on the surface. For example, on silver it will be chloride, on lead it will be sulfide. The film will help you easily separate the mold from the coating. In the case of copper, silver and lead, coat the surface with a 1% sodium sulfide solution to create insoluble sulfides.
Materials and equipment
When the mold is ready, place it in a galvanic bath connected to an electric current (to prevent the release film from dissolving). First, we coat the conductive copper layer under conditions of low current density.
We will need the following composition:
- copper sulfate - 150-200 grams;
- sulfuric acid - 7-15 grams;
- ethyl alcohol - 30-50 milliliters;
- water - 1 liter.
The operating temperature in the electrolyte bath is 18-25 degrees Celsius. Current density is from 1 to 2 Amperes per square decimeter. Alcohol will be needed to improve the wettability of the coating.
A car battery charger can be used as a DC source. We also need an ammeter with the ability to measure current from 0 to 3 or 5 amperes.
Usually the chargers already have an ammeter.
Nichrome wire will serve as a rheostat. We wind it on any ceramic plate. A coil from an electric heater will do just fine.
Any plastic container with a volume from 2 to 50 liters, depending on your needs, is suitable as a bath. We use a copper plate as an anode.
Note! The anode area should be approximately equal to the area of the workpieces.
To create a conductive layer for the product, add a few drops of varnish to the bronze powder. It is recommended to use colorless nitro varnish. The varnish needs to be made more liquid, so dilute it with acetone to the consistency of a liquid paint and varnish composition.
Manufacturing process
We take approximately a 20-centimeter piece of multi-core cable and remove the wire from it. We protect the insulation on both sides of the wire, bend one end of it at an angle of 90 degrees and glue it to the plastic part with instant glue. Moreover, BF glue will not work, since bronze paint will dissolve it.
When the items are dry, we degrease them using household chemicals (for example, washing powder). Next, rinse the product in running water or treat it with acetone.
The parts are firmly fixed to the wire. Now they can be dipped one at a time into pre-prepared bronze paint or this material can be applied with a brush. The entire surface must be evenly painted. It is recommended to use insulated wire from the cable, otherwise copper will fall on the bare wire, which will lead to additional consumption of the anode.
After drying the surface for an hour, twist the dried ends of the wires together. The parts must not touch each other. Next, we connect the products to the positive contact and immerse them in the bath. A few seconds after immersion, the copper plating process, visible to the naked eye, will begin.
The thickness of the copper coating may vary depending on the circumstances, but for small items it will be approximately 0.05 millimeters. The parts are in the bath for 15 hours.
The current is adjusted by moving the contact along the nichrome rheostat within 0.8-1.0 Amperes. After copper plating, we increase the current to 2 Amperes.
When the curing period of the parts has expired, we wash the items in running water, dry them, and cut off the wire. We clean the wire and prepare it for the next procedure.
Metallization is complete. Next, take sulfur ointment (can be purchased at a pharmacy), apply it to the surface and carry the part over the fire of a gas stove. In this case, the copper will immediately darken.
The next stage is polishing. For this, a motor equipped with a metal round brush is useful. This job requires a certain skill. The result should be a surface that looks like blackened bronze with some shiny areas. If you cannot immediately achieve the desired result, apply sulfur ointment again, heat the product over the fire and polish it.
For those who doubt the effectiveness of the procedure described above, we suggest doing a test. To do this, you will need a container for electrolyte, where you need to put a little copper. Paint one part with a spray bottle in 2-3 layers in bronze color. Next you need to connect to the battery without using a rheostat. The adapter from the player will also work.
In addition to copper, other metals can be applied to a non-metallic surface, including gold or silver. Silver electroplating can be carried out in one of two ways: chemical or electrochemical.
Chemical silvering is produced by immersing the product in a boiled solution of silver. The electrochemical process gives a more reliable result, since the coating is more durable as a result of exposure to electric current.
Silver electroplating is widely used in the production of jewelry.
So, electroplating at home is quite possible. The process is quite labor-intensive and requires certain skills, but the end result is worth it.
Galvanic treatment at home
Those who remember well the school curriculum for the Chemistry course will immediately answer the question of what electroplating is.
For those who have forgotten a little, let us remind you that this is a branch of electrochemistry, the so-called process when a metal coating is applied to almost any product.
This process is also used on an industrial scale, for example, both in the galvanizing or chrome plating of metal products, and in the manufacture of decorative items.
The process of deposition of electrolytes onto the desired surface is quite complex and requires compliance with safety precautions and certain home processing skills. Electroplating at home will not allow you to increase the strength of a metal product (this requires industrial capacity), but it can be used to decorate individual items.
Galvanic laboratory at home
To organize the process you will need:
- Do-it-yourself galvanic bath - a jar (made of glass or durable plastic, large enough to fit the product being processed, heat-resistant) with an electrolyte solution.
- A wire divided into an anode (“plus”) and a cathode (“minus”). In this case, the anodes must be larger in area than the product being processed. They conduct current into the electrolyte and replace the loss of metal in it, which will be deposited on the galvanized product.
- Weighing equipment, such as precision electronic scales.
- A DC power source with voltage regulation, a household outlet will not work.
- Electric stove with mandatory temperature control.
How to glue metal to metal
In everyday life, we are constantly forced to solve problems of varying degrees of economic complexity, like nailing a shelf, screwing in a light bulb, or fixing pipes.
During the process of repair work, many people had a question: how to glue metal to metal, without the help of welding or riveting. The mission seems impossible at first, but fortunately, the industry does not stand still and it is now possible to firmly glue metal to metal, as well as other materials (such as wood, plastic, glass, leather, aluminum and even paper) using specialized glue.
Glue for metal. How it works?
To each according to his needs! When it comes to gluing materials, we do not want to purchase low-quality adhesive mass, otherwise the whole point of the intended work will simply be lost.
When choosing an adhesive base, focus on the following product parameters:
- Water resistance. This quality is especially valuable if the glued surface is located in the open air.
- Long validity period.
- Availability of corrosion protection.
- Minimized shrinkage and expansion during curing.
- Resistance to changes in temperature conditions.
- Reliability of the material.
Most often, when purchasing glue for metal, they focus on such an indicator as viscosity. The higher the viscosity of the adhesive mass, the more versatile the product itself. If the viscosity is high, then thanks to this glue you can glue not only metals, but also other hard surfaces.
Before visiting a hardware store, you need to answer the following questions:
- Where exactly will the glued material be located?
- How firmly should the surface used be bonded?
- Are loads expected directly on the adhesive joint? What intensity will the expected loads be?
- The types of iron you want to connect.
- How exactly will the seam be formed?
- Will there be sudden temperature changes?
Once the answers to these questions have been found, you can safely start purchasing glue.
Remember that metal glue contains chemical compounds that can cause negative effects on your health. Before buying glue, inquire about its toxicity and pay special attention to the precautions indicated on the packaging. Neglecting basic safety rules can result in disastrous consequences for you and your loved ones.
How to glue metal to metal?
If you don’t know anything about welding, or you simply can’t afford it, but you really need to connect what appears to be incompatible at first glance, then glue for metal surfaces will save you. Metal adhesive has a number of chemical properties that make it a better alternative to welding. In addition to the “death grip”, bonded surfaces are not afraid of vibration and other aggressive influences.
When choosing an adhesive, you need to know exactly how and on what surface it will be used. There are a large number of narrow-profile adhesive masses on the market, which differ in their properties and areas of application. Let's take a closer look, so how do you glue metal to metal?
Epoxy adhesive. Cheap doesn't mean bad
Two-component epoxy adhesive is a truly universal material; it is used in everyday life, in industry and on a construction site; you cannot do without it. The price for this type of glue is relatively affordable, and the scope of use is wide.
Epoxy glue does not care about cold and heat, it is not afraid of water, gasoline and kerosene. Due to its durability, epoxy adhesive is widely used in outdoor work and for gluing materials that will be exposed to aggressive environmental influences.
Before you start using this type of glue, you need to prepare it. The adhesive mass initially consists of two parts: epoxy resin and hardener; after mixing these two components, you can begin to work.
If for any reason you need to improve the adhesive properties of epoxy glue, then you can add materials such as metal shavings or ceramics to the resulting mass. Thanks to such a “prefabricated” mass, it is possible to qualitatively restore the shape of various metal products.
Walking through the aisles of a hardware store and seeing a catchy name on epoxy glue like “cold welding,” remember that this is just an advertising gimmick, “real” cold welding is a method that involves the use of special mechanical means that deform the surfaces being joined in a special way.
One of the most popular varieties of this type of glue is: “Moment Super Epoxy Metal”. In addition to good frost and heat-resistant qualities, this product is perfect for applying neat seams.
Cold welding for metal
One of the most proven methods of joining metals is the so-called “cold welding”. The cold welding technique is based on the use of deformation processes that occur during compression or sliding of the materials being joined. This type of connection of solid bodies has been known to mankind for a long time, since our distant ancestors connected gold and other metals with powerful blows.
In the process of development of the chemical industry and construction, the concept of “cold welding” has undergone changes and now cold welding is most often understood as a specialized construction adhesive, which is valuable because it retains its properties even at very high temperatures.
Scotch tape for metal
One of the most popular methods of joining iron is industrial tape, which is designed for quick, relatively safe and reliable gluing of hard materials. The base of such adhesive tape is based on polypropylene or a foam base on both sides of which an adhesive is applied.
Double-sided tape is practically irreplaceable in everyday life; it can be used to easily glue towel hooks or a car rear-view mirror. An article on the topic of how to wipe off traces of tape.
Adhesive materials - “PERMABOND”
How else can you glue iron to iron? You can try adhesive materials from the PERMABOND company; they will solve your question about how to glue metal to metal. The products of this company are based on methyl cyanoacrylate, which has the remarkable property that it does not spread, which means that the joints and the entire adhesive surface will be smooth and neat.
Anaerobic adhesive for metal
The scope of application of this product is quite narrow. Anaerobic glue is mainly used for gluing threads and bushings; when working with systems such as “shaft-bearing” and “shaft-bushing”, in addition to these works, the glue can be used as a sealant.
A special feature of working with this material is that upon contact with oxygen, the material creates an airtight film, inside which the hardening process occurs. When choosing this material, please note that it comes in several types, which differ in viscosity and adhesion strength.
Cyanoacrylate glue
It is truly “instant glue.” The curing time of the adhesive mass of this product is only 5 seconds at room temperature. In addition to being instantaneous, this glue has good strength and retains its properties perfectly both at low and high ambient temperatures. This glue is perfect for joining small metal parts.
When using this product, remember that it does not involve “correction” of the material, that is, the glued surface will not be able to move.
Methyl methacrylate glue
This glue is quite rarely found in ordinary construction stores. Its main area of use is automotive industry. This narrow specialization is due to the fact that this product has excellent impact resistance, which is especially important in the production of parts for motor vehicles.
How to glue metal to metal? Instructions
So, you are serious about starting to glue metal products. But before you glue metal to metal, choose an adhesive that suits the task at hand.
Choosing the right adhesive
- If you need to connect fairly large metal parts and cannot do without overlap, epoxy glue is ideal.
- Do you want to connect “back to back”, that is, glue small parts together? Use adhesives containing cyanoacrylate.
- Do you need to restore a thread? Feel free to buy anaerobic glue.
- If you need the “gluing” to happen as quickly as possible, cyanoacrylate glue will help.
- In a situation where you can have the right to correct mistakes, you need epoxy glue, which does not harden immediately and makes it possible to move the adhesive surface.
Preparing the adhesive surface
After the glue is selected, it is necessary to prepare the surface to be used and get rid of rust on the metal. Thorough cleaning allows the glue to adhere better, which means the result will be more reliable. You can clean the surface using materials such as sandpaper or a brush with metal bristles. It is worth noting that when cleaning coarse dirt on large surfaces, it is permissible to use a grinder with an abrasive brush.
Not a drop of fat! After cleaning is completed, it is necessary to degrease the surface used as much as possible. Depending on the parameters of the material, ordinary cotton wool or rags pre-moistened in acetone can be used for this purpose.
Glue preparation
If the glue requires preliminary preparation (for example, to obtain epoxy glue, you must first mix its components), then use a plastic bucket to make it, mix the mass, preferably with a plastic spatula.
Applying adhesive mass
At this stage, focus on your needs. On large, voluminous materials, it is best to apply glue using a plastic spatula, and when gluing small parts, you can get by with an ordinary match.
Protected from humidity
During the hardening process of the adhesive mass, try to avoid exposure to moisture on the glued surface. If the glued surface will be constantly exposed to “fresh air”, then it would be best to additionally cover it with a water-repellent solution. If the gluing process will take place outside, stock up on an awning or umbrella that will protect the uncured surface from excess moisture.
The ideal climatic option for the work being carried out would be room temperature with minimal air humidity.
Safety precautions
By following these simple instructions on how to glue metal to metal, you will achieve the desired gluing quality. During work, it is best to protect yourself from direct inhalation of vapors from the hardening adhesive mass, since they are the ones that have the maximum toxicity.
The ideal and safest option would be to use a special respirator, which can also be purchased at a hardware store. Remember, your safety is in your hands.
How to remove glue from metal?
- The first type of the most effective means for cleaning metal from old glue is considered “Super Moment Anti-Glue”. It will help you completely get rid of universal PVA glue, instant glue and various polyurethane-based adhesives. The gel base allows it to be used even on a vertical surface.
- The second option is to use the pharmaceutical product "Dimexide". It is necessary to thoroughly moisten a cotton wool or cloth in this composition and treat the surface until the adhesive stain completely disappears.
- Not least in removing glue are such common products as: “White Spirit”, acetone, the cleaning component “Contact” and, of course, gasoline.
- Manicure and pedicure professionals use emulsions to soften acrylic. They will effectively help dissolve super glue from the metal surface.
It's important not to forget! Fresh stains of any glue can be removed much easier than old (dried) ones. So be careful and extremely careful when working with any adhesive compositions.
Keeping up with progress
Modern adhesive materials for metal surfaces allow almost any consumer to glue metal to metal without any problems and costs, without using welding. Thanks to its ease of use and good results, you can independently recreate the integrity of small and medium-sized parts at home and carry out construction work without resorting to the services of a welder.
Remember that most of your success in gluing metals depends on the correct choice of adhesive product and on following the technology for working with this easy-to-use material.
In addition to excellent adhesive properties, you must remember that you are dealing with a product that can emit toxic fumes, so always use additional protective measures when working. We hope that a properly glued surface will serve you for a very long time.