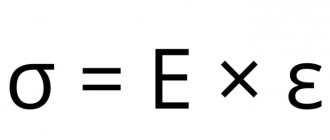Physics Glossary
|
Strength of solids in a broad sense
— the ability of solids to resist destruction (separation into parts), as well as irreversible changes in shape (plastic deformation) under the influence of external loads.
Strength of solids in the narrow sense
- resistance to destruction. Depending on the material, the type of stress state (tension, compression, bending, etc.) and operating conditions (temperature, load duration, etc.), various measures of the strength of solids (yield strength, tensile strength, fatigue limit) are used in technology etc.).
The destruction of a solid body is a complex process that depends on many factors, therefore the values that determine the strength of solid bodies are conditional.
Rice. 1. Dependence of the force of interaction between two atoms on the distance between them.
Physical nature of strength
The strength of solids is ultimately determined by the interaction forces between the atoms or ions that make up the body. For example, the strength of interaction between two neighboring atoms (if we neglect the influence of surrounding atoms) depends only on the distance between them (Fig. 1). At an equilibrium distance r0 ~ 0.1 nm (1), this force is zero. At smaller distances the force is positive and the atoms repel, at larger distances they attract. On critical distance force of attraction in abs. the value is maximum and equal to FT. For example, if when stretching a cylinder-rich. rod with cross section S0, the acting force P directed along its axis is such that the external force per given pair of atoms. force exceeds max. force of attraction FT, then the atoms move away from each other without hindrance. However, for a body to collapse along a certain surface, it is necessary that all pairs of atoms located on both sides of the surface in question experience a force exceeding FT. The voltage corresponding to the force F t is called. theoretical tensile strength s t (sT0.1 E, where E is Young’s modulus). However, in practice, destruction is observed under load P*, which corresponds to a voltage s = P*/S 100-1000 times less than s t· Discrepancy between the theoretical. P. t. t. with real is explained by inhomogeneities in the structure of the body (grain boundaries in polycrystalline material, foreign inclusions, etc.), due to which the load P is distributed unevenly over the cross section of the body.
Mechanism of destruction
If on a surface area of small dimensions (but significantly larger than the cross section of one atom) the local stress is greater than s t, a rupture will occur along this area. The edges of the rupture will diverge to a distance greater than rк, at which point the interatomic forces are already small, and a microcrack will form (Fig. 2). The nucleation of microcracks at stress below st is promoted by thermal. fluctuations.
Rice. 2. Griffith crack; The area in which stress is relieved is shaded. Arrows indicate the direction of voltage.
Local stresses are especially high at the edge of the formed crack, where stress concentration occurs, and the larger the crack size, the greater they are. If this size is more than a certain critical. rс, the atoms at the edge of the crack are subject to a stress exceeding st, and the crack grows further along the entire cross-section of the body at high speed - destruction occurs. The value of rc is determined from the condition that the elastic energy of the material released during crack growth covers the energy costs for the formation of a new crack surface: (where g is the energy per unit surface of the material). Before the increasing ext. the force will reach the value necessary for destruction, dep. groups of atoms, especially those that are part of defects in crystals, usually experience rearrangements, during which local stresses decrease (“relax”). As a result, an irreversible change in the shape of the body occurs - plasticity. deformation; it is also facilitated by thermal. fluctuations. Fracture is always preceded by greater or lesser plasticity. deformation. Therefore, when estimating rc, the energy g must include the work of the plastic. deformation ur. If plastic the deformation is large not only near the fracture surface, but also in the volume of the body, then the fracture is viscous. Destruction without noticeable traces of plastic. deformations called fragile. The nature of destruction is manifested in the structure of the fracture surface. In crystalline In bodies, brittle fracture corresponds to crystallographic chipping. cleavage planes, viscous - merging of microvoids and sliding. At low temperatures, destruction is predominant. brittle, at high - viscous. The rate of transition from ductile to brittle fracture is called. critical cold brittleness temperature.
Since fracture is the process of nucleation and growth of cracks and pores, it is characterized by the speed or time from the moment the load is applied to the moment of rupture, i.e., the durability of the material. Research of many crystalline and amorphous bodies showed that in a wide range of temperatures T and stresses s applied to the sample, tensile durability is determined by the relation
where approx. equal to the period of thermal vibrations of atoms in a solid (10-12 s), the energy U0 is close to the sublimation energy of the material, activation. volume V is usually several. thousand atomic volumes and depends on the structure of the material formed during the preliminary thermal process. and mechanical processing and during loading. At low temp-pax, the durability drops very sharply with increasing stress, so that for any value important for practice, there is an almost constant limiting value of stress above which the sample is destroyed almost instantly, and below it it lives indefinitely. This value of s0 can be considered the strength limit (table).
Tensile strength values
kgf/mm2 (1 kgf/mm2=10 MN/m2)
| s0 | s0/E | |
| Graphite (filamentary crystal) | 2400 | 0,024 |
| Sapphire (threaded crystal) | 1500 | 0,028 |
| Iron (whisker) | 1300 | 0,044 |
| High Carbon Steel Drawn Wire | 420 | 0,02 |
| Tungsten drawn wire | 380 | 0,009 |
| Fiberglass | 360 | 0,035 |
| Mild steel | 60 | 0,003 |
| Nylon | 50 |
Time is spent waiting for thermal fluctuations. the initiation of microcracks and their growth to critical. size . When a stress s is applied to a sample, it deforms first elastically, then plastically, near the structural inhomogeneities that were present in the initial state or that arose during plasticity. deformation, large local stresses are formed (for example, in crystals - as a result of the accumulation of dislocations). Microcracks originate in these places. Their concentration can be very high (for example, in some polymers there are up to 1015 cracks per 1 cm3). However, their sizes, determined by the scale of structural heterogeneities, are significantly smaller. Under the post. under stress, the size and concentration of cracks grow slowly and the body does not collapse until by chance (for example, as a result of the sequential merging of closely spaced adjacent cracks) one of them grows to critical. size. Therefore, when creating durable materials, care should be taken not so much to ensure that cracks do not appear, but rather to ensure that they do not grow.
Random distribution of structural inhomogeneities over the volume of the sample, in size and in degree of strength and the random nature of the term. Fluctuations lead to a scatter in the durability values (as well as the P.T.T. limit) when testing identical samples at given values of T. The greater the probability of encountering a “weak” point in a sample, the larger its volume. Therefore, the PT (breaking stress) of small samples (for example, thin threads) is higher than that of large ones made of the same material (the so-called scale effect). Areas with increased stress, where microcracks nucleate more easily, are more common near the surface (protrusions, scratches). Therefore, surface polishing and protective coatings increase P. t. t. On the contrary, in aggressive environments P. t. t. is reduced.
Rating of the strongest elements in the world
There are a large number of well-known metals and alloys. Among the strongest are 10 elements.
Tantalum
A metal called tantalum, which was discovered in 1802, ranks third on our list. It was discovered by the Swedish chemist A. G. Ekeberg. For a long time it was believed that tantalum is identical to niobium. However, the German chemist Heinrich Rose managed to prove that these are two different elements. Scientist Werner Bolton from Germany was able to isolate pure tantalum in 1922. This is a very rare metal. The largest deposits of tantalum ore were discovered in Western Australia.
Due to its unique properties, tantalum is a highly sought-after metal. It finds a variety of uses:
- In medicine, tantalum is used in the production of wires and other components that can bind tissue and even act as a bone substitute;
- Alloys containing tantalum are resistant to aggressive environments and are therefore used in the aerospace and electronics industries;
- Tantalum is also used to produce energy in nuclear reactors;
- It is also widely used in the chemical industry.
Titanium
The last place in the top ten hardest metals is titanium. The first pure form of this element was obtained by the chemist J. J. Berzelius of Sweden in 1825. Titanium is a lightweight, silver-white titanium metal that is very hard and resistant to corrosion and mechanical stress. Titanium alloys are used in many branches of mechanical engineering, medicine and the chemical industry.
Iridium
Iridium is at the top of the list of the hardest metals. It was discovered at the beginning of the 19th century by the English chemist Smithson Tennant. Iridium has the following physical properties:
- It has a silvery-white color;
- Its melting point is 2466 oC;
- Its boiling point is 4,428 °C;
- Its resistivity is 5.3-10-8 Ohm-m.
Because iridium is the hardest metal on the planet, it is difficult to work with. However, it is still used in various industrial applications. For example, it is used to make small balls that are used in pens. Iridium is also used in the production of components for space rockets and some automotive parts.
Very little iridium occurs in nature. Findings of this metal are a kind of proof that meteorites fell where it was found. These cosmic bodies contain significant amounts of this metal. Scientists believe that our planet is also rich in iridium, but its deposits are closer to the Earth's core.
Tungsten
The hardest metal found in nature. This rare chemical element is also the most refractory of the metals (3422 °C).
It was first discovered as an acid (tungsten trioxide) in 1781 by Swedish chemist Carl Scheele. Further research led two Spanish scientists, Juan José and Fausto d'Elhujar, to the discovery of acid from the mineral tungramite, from which tungsten was then isolated using charcoal.
In addition to its widespread use in incandescent lamps, tungsten's ability to perform under extreme thermal conditions makes it one of the most attractive elements for the weapons industry. During World War II, metal played an important role in establishing economic and political relations between European countries.
Tungsten is also used to produce carbide and in the aerospace industry to produce rocket nozzles.
Beryllium
Now it’s better not to protect this metallic beauty. Because beryllium is very toxic and also carcinogenic and causes allergies. If you breathe air containing beryllium dust or beryllium fumes, you may develop beryllium, a disease that affects the lungs.
However, beryllium is not only harmful, but also useful. For example, add just 0.5% beryllium to steel, and you will get springs that will be elastic even when brought to red heat. They can withstand billions of load cycles.
Beryllium is used in the aerospace industry to create heat shields and guidance systems, and to create fire-resistant materials. Even the vacuum tube of the Large Hadron Collider is made of beryllium.
Uranus
This naturally occurring radioactive substance is very widespread in the earth's crust, but is concentrated in certain hard rocks.
One of the hardest metals in the world, it has two important commercial uses - nuclear weapons and nuclear reactors. Therefore, the end products of the uranium industry are bombs and radioactive waste.
Rhenium
Rhenium is a very rare and expensive metal that, although found naturally in its pure form, is usually added to molybdenite.
If Iron Man's suit were made of rhenium, it could withstand temperatures of 2000°C without losing strength. What will happen to Iron Man inside the suit after such a “fire show” is kept silent.
The metal is used in the petrochemical and chemical industries. This metal is used in the petrochemical industry, electronics and electrical engineering, and in aircraft and rocket engines.
Osmium
Silvery, bluish metal of light color.
It belongs to the platinum group and is considered one of the densest elements. It is characterized by hardness. Os is a brittle metal, but it is resistant to mechanical stress and acid-resistant. Scientists have recorded the presence of osmium in metal meteorites. Forming an ideal composition with other elements, it is widely used in medicine, electronics, chemistry and petrochemistry, rocket science, and is widely used in the production of pens.
Chromium
Chrome is a blue-white metal. It has high strength and hardness, as well as strong magnetic properties. It does not become brittle and is resistant to acids and alkalis.
It is used in the production of various alloys that are used in medical equipment. Cr is also used in the synthesis of artificial rubies, and chromium salts are used for wood preservation and leather tanning.
Ruthenium
The name of the second most powerful metal in the ancient language, ruthenium, means Russia. This metal has a silvery color, belongs to the platinum group and is found in the muscle tissue of all living creatures on earth.
It is a high-strength, hard, refractory metal, resistant to chemicals and capable of forming complex compounds. Ruthenium is used in the aerospace, medical and electronics industries, and as an additive to give gold its black color.
Graphene
Molecular lattice of graphene. The first item on our list is a material that is widely used in the aerospace and automotive industries. When safety comes first and launching rockets into space seems very dangerous, the use of graphene is simply necessary. It is 200 times stronger than steel. Graphene consists of a single layer of carbon atoms arranged in a triangular lattice.
Iron and steel
As a pure substance, iron is not as hard compared to other participants in the rating. However, due to the minimal cost of extracting it, it is often used in combination with other elements to produce steel.
Steel is a very hard alloy made from iron and other elements such as carbon. It is the most commonly used material in construction, mechanical engineering and other industries. And even if you have nothing to do with them, you still use steel every time you cut food with a knife (unless it's ceramic, of course).
Artificial metal
In 2015, Californian scientists created microplates. It is currently the lightest metal on Earth, consisting of 99.99% air. However, due to its special design, the element has high strength. It is an interweaving of tubes, each of which is the size of 0.001 human hair. The amazing properties of microfiber are just beginning to be fully exploited in industry.
Carbon fiber
Black carbon fiber composite. The characteristics of carbon fiber that make it an excellent choice for military vehicles, missiles, and sports car parts are its high stiffness and very low weight. Carbon fiber can also withstand very high temperatures and is highly resistant to a variety of abrasive chemicals. Essentially, carbon fiber is super-dense, aligned carbon atoms that are 5 to 10 micrometers in diameter.
Mechanical properties of metals
Strength of metals
Strength
- the property of solids that resists destruction, as well as irreversible changes in shape. The main indicator of strength is the temporary resistance, determined at the rupture of a cylindrical sample that has been previously annealed.
Based on their strength, metals can be divided into the following groups:
weak metals
- (temporary resistance does not exceed 50 MPa) - tin, lead, bismuth, as well as soft alkali metals.
durable metals
- (from 50 to 500 MPa) - magnesium, aluminum, copper, iron, titanium and other metals that form the basis of the most important structural alloys
high strength metals
— (more than 500 MPa) — molybdenum, tungsten, niobium, etc.
The concept of strength does not apply to mercury, since it is a liquid.
The tensile strength of metals is indicated in Table 10.
Table 10. Strength of metals
| Metal | Tensile strength, MPa | Metal | Tensile strength, MPa |
| Titanium | 580 | Zinc | 120-140 |
| Iron | 200-300 | Aluminum | 80-120 |
| Copper | 200-250 | Gold | 120 |
| Magnesium | 120-200 | Tin | 27 |
| Silver | 150 | Lead | 18 |
Plasticity of metals
Plastic
- the property of solids to retain part of the deformation when the loads that caused them are removed. As an indicator of ductility, relative elongation is selectively determined by the same tests as tensile strength.
According to the degree of ductility, metals are usually divided as follows:
highly plastic metals
- (relative elongation exceeds 40%) - metals that form the basis of most structural alloys (aluminum, copper, iron, titanium, lead) and “light” metals (sodium, potassium, rubidium, etc.)
ductile metals
- (relative elongation ranges between 3% and 40%) - magnesium, zinc, molybdenum, tungsten, bismuth, etc. (the most extensive group)
brittle metals
- (relative elongation less than 3%) - chromium, manganese, colbate, antimony.
High purification of brittle metals slightly increases ductility. Alloys obtained from them are almost impossible to process under pressure. Industrial products from them are often produced by casting. The relative elongation of metals is characterized in Table 11.
Table 11. Plasticity of metals
| Metal | Relative extension, % | Metal | Relative extension, % |
| Gold | 65 | Titanium | 50 |
| Silver | 65 | Tin | 40 |
| Lead | 65 | Aluminum | 30-40 |
| Copper | 50-60 | Zinc | 30 |
| Iron | 40-50 | Magnesium | 10-22 |
Hardness
Hardness
- a characteristic of a material that reflects its strength and ductility, determined by indenting a ball (Brinell method) or a prism (Vickers method). A quantitative assessment of hardness is the hardness number HB, equal to the ratio of the load (N) to the surface area of the print (mm2).
The Brinell hardness values of metals are given in Table 12.
Table 12. Hardness of metals
| Metal | NV | Metal | NV |
| Titanium | 160 | Aluminum | 16-25 |
| Iron | 70-80 | Silver | 25 |
| Magnesium | 30-40 | Gold | 18 |
| Copper | 40 | Tin | 5 |
| Zinc | 33 | Lead | 4 |
Modulus of longitudinal elasticity
Modulus of longitudinal elasticity, Young's modulus, E
— determines the hardness of the metal, i.e. the intensity of the increase in stress as the elasticity of the deformation increases.
Table 13. Young's modulus of metals at 20 oC
| Metal | E * 10-5, MPa | Metal | E * 10-5, MPa |
| Iron | 2,17 | Gold | 0,83 |
| Zinc | 1,30 | Aluminum | 0,72 |
| Copper | 1,25 | Tin | 0,55 |
| Titanium | 1,08 | Magnesium | 0,45 |
| Silver | 0,83 | Lead | 0,18 |
Strength Literature
- Gul V. E., Structure and strength of polymers, 3rd ed., M., 1978;
- Destruction, trans. from English, vol. 1, M., 1973;
- Regel V.R., Slutsker A.I., Tomashevsky E.E., Kinetic nature of the strength of solids, M., 1974.
to the library to the table of contents FAQ on ethereal physics TOEE CHPP TPOI
Did you know,
How is Olbers' paradox resolved? (The photometric paradox, Olbers' paradox, is one of the paradoxes of cosmology, which consists in the fact that in a Universe uniformly filled with stars, the brightness of the sky (including the night sky) should be approximately equal to the brightness of the solar disk. This should take place because, according to any direction of the sky, the ray of vision will sooner or later hit the surface of the star. In other words, Olbers' paradox is that if the Universe is infinite, then we will not see a black sky, since the radiation of distant stars will be summed up with the radiation of nearby ones, and the sky should have an average temperature of the photospheres stars. When light is absorbed by interstellar matter, it will heat up to the temperature of stellar photospheres and radiate as brightly as stars. However, the phenomenon of “light fatigue” comes into play, discovered by Edwin Hubble, who showed that the farther a galaxy is located from us, the more the light of its radiation turns red, that is, the photons seem to “get tired”, giving up their energy to the interstellar medium.At very large distances, galaxies are visible only in the radio range, since their light has completely lost energy while traveling through the vast expanses of the Universe. Read more in the FAQ on ethereal physics.
The strongest metal alloy in the world
Platinum + gold - the world's hardest metal alloy
And straight to the heart of the scientific achievement - scientists from Sandia National Laboratory in the USA have developed a new metal alloy and called it the strongest alloy ever created by scientists in laboratories around the world. The newly developed material, made from a combination of platinum and gold, is estimated to be 100 times stronger than high-strength steel, making it the first metal alloy in the same class as diamond surfaces.
Plasticity characteristics
Relative elongation is the difference between the initial and final length of a tensile sample, showing the ability of the metal to plastically deform until failure. Metals with the same tensile strength may have different elongations. For example, for malleable cast iron grade KCh50-5 this figure does not exceed 5%, and for structural steel 09G2S it reaches 20% with a tensile strength equal to 490 MPa for both materials.
The metallurgical industry always strives to create high-strength metal materials without loss of ductility, selecting optimal chemical compositions of steel, and improving production technologies. To achieve high mechanical properties, while maintaining the same composition and volume of the product, unique modes of smelting, mechanical, thermal, chemical-thermal treatment are selected to create a homogeneous, fine-grained, clean and defect-free steel structure.
Yield strength
The most interesting parameter is the yield strength. At the beginning of the test, when the sample begins to stretch, the deformations in its structure are reversible. That is, if you stop stretching before a certain point, the sample under study will return to its previous state due to elastic deformation.
However, after reaching the “point of no return,” the metal can no longer elastically return to its original dimensions—irreversible plastic deformation begins. The voltage at which this occurs is recorded by equipment, and is subsequently taken into account when describing the strength characteristics of the sample.
It is interesting that when calculating load-bearing structures, engineers mainly rely on the yield strength, and not on the tensile strength of the metal.