One of the most interesting materials produced in metallurgy is Hadfield steel. This is the first alloyed, high-manganese steel in mass production and active use. Because of its unusual properties, it is used in those areas of the national economy where all other types of steel are not suitable. It can quite rightly be called super steel. It has low hardness, but good wear resistance to impacts, high pressure and temperature changes. This steel is suitable for use in aggressive environments and extreme conditions.
Alloying
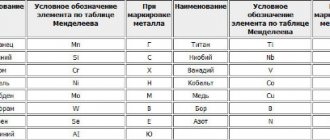
Alloying is a change in the composition of steel using a calculated amount of auxiliary elements, impurities, to give it certain physical qualities. Among the most commonly used alloying components are:
- manganese,
- titanium,
- cobalt,
- tungsten,
- aluminum,
- nickel,
- chromium,
- silicon,
- vanadium,
- niobium.
All these additives have different effects on the final qualities of the resulting alloy. Before purposefully adding alloying components to metal, people became acquainted with natural alloyed alloys that literally fell from the sky in the form of iron meteorites. This iron has been used for a long time. It contains up to 8.5% nickel, an alloying element actively used today.
This type of steel was invented in 1882 by the English metallurgist Robert Hadfield (he was accepted as an honorary member of the USSR Academy of Sciences in 1933). This is a highly ductile steel with a high manganese content. This brand of steel turned out to be so successful that even now, with virtually no changes in the chemical composition, it is widely used in a wide variety of industries. In the USSR, the technology for smelting this steel was mastered by 1936. In Russia and among member countries of the Commonwealth of Independent States, it is known under the brand name 110G13L (or G13L). The letter “L” means that this steel is for casting. The requirements are regulated by GOST 977-88 and its analogues abroad.
Designations
| Name | Meaning |
| Designation GOST Cyrillic | 110G13L |
| Designation GOST Latin | 110G13L |
| Translit | 110G13L |
| By chemical elements | 110Mn13 |
| Name | Meaning |
| Designation GOST Cyrillic | G13L |
| Designation GOST Latin | G13L |
| Translit | G13L |
| By chemical elements | Mn13 |
Application area
It is used to make parts of mechanisms, rail crosspieces, turnouts, cores for rolling pipes, caterpillar tracks, armor plates, parts of crushers, visors of dredging machines, all devices that require special resistance to wear at high pressures, shock loads and abrasion. Until the 1980s, it was used to make protective helmets for soldiers in the British and American armies. During the twentieth century, about 30 million of them were produced. These helmets are just one way Hadfield steel is used. In the 1920s, tracks for tanks began to be made from it - this is the part of the tracks that is subject to the greatest impact and abrasion when moving heavy vehicles. Made from this steel, they made it possible to increase the mileage of equipment without repairing tracks or replacing them by almost 10 times, from 500 km to 4800 km.
Hadfield steel is very important, it has become indispensable in the military industry and tank building. Over time, this type began to be used in other areas of activity.
Story
Hadfield steel, as one might guess from its very name, was obtained by a certain Hadfield. But few people know who this man is and when this steel was offered to him. If you delve into history, you can find out the fact that this material of increased strength was obtained by the English metallurgist Robert Hadfield in 1882 . It became widespread quite quickly, as it turned out to be very interesting material.
Soon after Hadfield steel was released into mass production, the military became interested in it. And not by chance. High strength steel was to become a very important material for the creation of some elements of protective military equipment.
The introduction of Hadfield steel into the military industry began with the material being used to create high-strength infantry helmets. At first, such helmets were adopted by the British army, but the American military did not stand aside and also began producing similar helmets. The technology for creating helmets from Hadfield steel remained unchanged until the 80s. The fact is that by that time, steel, which had already become famous, was replaced with organoplastic, since it was lighter, but remained quite durable.
Hadfield steel was also widely used in tank building . It was used to make caterpillar tracks for tanks. Hadfield steel was first used for this purpose from Britain in the late 20s. The use of this steel in the manufacture of tank tracks made it possible to significantly increase the mileage to 4,800 km. By the way, before using the miracle of steel, the mileage was 500 km. What’s most interesting is that this small figure was considered a real record during the First World War. This fact shows how important the use of Hadfield steel was in the field of tank construction.
Subsequently, the use of Hadfield steel was carried out not only in military fields. In the Soviet Union, smelting at this cost was mastered only by 1936.
Composition and properties
The percentage chemical composition of Hadfield steel is:
- Fe - 82%,
- Mn - 11.5-15%,
- C - 0.9-1.6%,
- Si - 0.3-1%,
- other components - up to 5%.
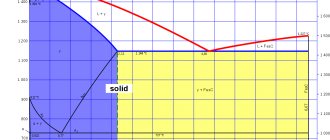
With this percentage of manganese and carbon, the steel has an austenitic structure. It is this that gives the metal increased resistance to wear and a tendency to increase strength when the geometry of the original shape is violated as a result of an impact. Austenite is the structure of a metal that determines its technical characteristics, which cannot be obtained in another state, since when the structure changes, the properties also change. This is a solid solution of carbon and alloying components in iron. The amount of carbon and the amount of manganese in the alloy are directly related. As the amount of carbon increases, the manganese content must also increase. The service life of protective coatings made from Hadfield steel (linings) depends precisely on the amount of carbon in the metal. Since manganese is an active metal, Hadfield steel has an increased weakness to corrosion; this is a significant drawback of this alloy.
Products made from this steel require special care to protect against corrosion damage.
Hardening
Impact hardening, or work hardening, is a process used to increase the strength of a metal, which cannot be obtained by thermal treatment (quenching). This processing technology is aimed at changing the shape of a product using cold forging, plastic deformation, and introducing mechanical energy into the metal. As a result, the hardness of the alloy increases, its strength increases, but its ductility decreases.
And the impossibility of hardening Hadfield steel to obtain the usual effect - strengthening the hardened part - was noticed by the inventor of this type of metal himself. When trying to harden the sample, it turned out that the metal became softer, not harder. Replacing the quenching media did not help; the sample remained soft. It was also unexpected that the new steel could not be turned or milled. When trying to forge the sample cold, without heating, the areas subjected to hammer blows became hard. And the more blows they received, the harder they became. An attempt to process the metal with a file also ended in failure. The stronger the pressure of the file, the stronger the resistance of the metal, the sample became more and more hard.
Because it is impossible to saw through a Hadfield steel bar with a file, it is used to make prison bars. When you try to cut a bar of such a grating, strong hardening occurs on the part that is exposed to the impact. The hardness of the steel increases significantly, up to the hardness of the file itself and even higher. As a result, an attempt to saw through the prison bars is doomed to failure.
Hadfield steel. Unwavering endurance.
Steel grade 110G13L is well known to heavy industry professionals. It is also known to people far from industry, although under a more euphonious name – “Hadfield steel”. And even in a non-professional environment they have heard about the impressive hardness of the alloy. The main “secret” of the special characteristics of this steel is its main alloying element – metallic manganese.
Swedish mineralogist Johan Gottlieb Hahn was the first to isolate metallic manganese in 1774 by heating pyrolusite ore with coal in a furnace. The first to add a new element to steel was the British metallurgist Robert Abbott Hadfield in 1882. Today manganese steel has the same chemical composition and bears the name of its inventor.
The metallurgist immediately realized that the new metal was fundamentally different from all the others: Hadfield tried to harden the sample, but the steel did not become harder, like all steels after hardening, but softer. Further more. The new metal was not amenable to either turning or milling. The scientist tried to harden steel in various environments, but it stubbornly became soft during processing.
When it was finally subjected to cold forging, an unexpected feature emerged: the areas where the hammer struck became hard. The greater the degree of deformation, the harder the steel became. Steel produced a similar effect when processed with a file. As pressure was applied, the resistance of the metal increased: the stronger the pressure, the greater the resistance.
The unique hardness and wear resistance of the new steel, its ability to withstand and absorb strong impacts without destruction, became a passport to the world of large industry. They began to use it for the manufacture of those parts that are constantly exposed to strong impacts and quickly fail. The first use of Hadfield steel was found by railroad builders. Rail crosspieces and turnouts operating under conditions of shock loads and abrasion were made from it.
Soon the military also became interested in the durable alloy. During the First World War, infantry helmets were made from Hadfield sheet steel for the British and American armies. Helmets served the armed forces under the Stars and Stripes faithfully until the 1980s. During the twentieth century, the military produced about 30 million helmets made of manganese steel.
Having appreciated the merits of the alloy on helmets, the military gave it the green light for larger-scale projects. In the 1920s, the use of metal in tank construction began. The first to use metal for tank tracks was the British company Vickers. The new material made a real breakthrough: now combat vehicles could withstand almost 5,000 kilometers - 10 times more than before.
Perhaps the most unusual, but quite natural application of manganese alloy was prison window bars. Forget all the movies in which the hero-prisoner literally saws his way to freedom. You won’t be able to do this trick with a Hadfield steel grate - during sawing, the surface being processed is strongly hardened - the metal becomes as hard as the saw and even harder.
Today, Hadfield steel is most often used in the mining and mechanical engineering industries. Due to its high hardness, significant toughness and phenomenal abrasion resistance, steel is used for working parts of crushers, mills, tractor and machine tracks, excavator buckets and, in general, all parts subject to strong mechanical stress. All products made from such steel have a very long service life.
Steel owes its excellent consumer qualities, as we have already said, to its chemical composition. According to the classic “recipe” the alloy contains 13% manganese and 1% carbon. Manganese removes sulfur and oxygen impurities from the metal and contributes to the saturation of steel with carbon and nitrogen. And with such proportions of elements in the composition, steel has an austenitic structure. It is this that provides increased resistance to wear and strengthening during deformation. Manganese steel boasts colossal hardening - the hardness of the alloy reaches 80 Rockwell units.
Hadfield steel is one of the most useful and sometimes irreplaceable materials. It is used in areas where the use of other steel is sometimes simply impossible.
Source
What is hardening?
Hardening is an increase in the strength of metals and alloys due to changes in their structure during plastic deformation at a temperature below the recrystallization temperature. That is, the temperature at which, in place of elongated metal grains that have lost their shape, new grains with an undistorted lattice and regular round shape begin to appear and grow. When a metal is hardened, its density decreases; this occurs due to a violation of the order in the arrangement of atoms, distortion of the atomic lattice, the formation of micropores, and an increase in the density of defects. A decrease in density means an increase in the specific volume per unit mass. The outer hardened layer tends to expand, but the inner ones do not allow it to do so. Residual compressive stresses arise in the metal. They can be very useful, as they are able to stop the process of appearance and increase in surface fatigue cracks.
It is impossible to guarantee uniform constancy of specific pressures in the hinge in the range from 80 to 200 kg/cm, at which the ability of steel to work hardening is manifested, and thereby its ability to resist wear is revealed. Below these indicators, hardening of Hadfield steel is not observed, and above this, residual deformation occurs, and accordingly, its abilities cannot be fully used. Numerous observations of the operation of STZ NATI tractors in the field have shown that after about a thousand hours of operation, the wear of the eye holes of the hinge joints is 0.3 - 0.4 cm, and as a result of one and a half to two thousand hours of operation, the eyes are worn out to almost the entire thickness of the wall 0, 8 cm or destroyed earlier.
Changing alloy properties
When a metal is subjected to mechanical stress, microscopic defects are formed in it - dislocations; if such impact continues, these defects begin to shift and interact. They form a new material structure that resists further plastic change in shape. This structure increases the metal's ability to resist applied forces, increases the material's yield strength and reduces its viscosity. This is very important for those metals and alloys that are not strengthened during heat treatment.
At room temperature, Hadfield steel is practically non-magnetic, but after cold deformation, magnetic properties appear. This phenomenon is accompanied by the appearance in the metal structure of dense slip planes of dislocations, which crush the grains into separate blocks. The discovery of Hadfield and Hopkinson was that tensile testing of a steel sample gave it weakly magnetic properties. The appearance of ferromagnetism shows that after this type of load, part of the metal passes into the a-iron state.
Processing methods
Cold forming of metals is a well-known method of intentionally creating hardening. Typical technological processes for such metal processing are drawing, cold forging, rolling, pressing (extrusion). If you overdo the processing, the Hadfield steel part can fall apart due to increasing internal stresses that destroy it. Therefore, when processing, for example, a knife blade, which is recommended to be slightly beaten before final sharpening, or when beating a braid (and this is cold forging), you need to apply very light blows and be careful about the recoil from the hammer. As soon as it starts to bounce, it's time to stop hitting, otherwise the blade may crumble.
Due to the high viscosity of Hadfield steel, parts made from it practically cannot be processed with cutting tools. For mass production of products from this steel, only casting is suitable. Casting molds must be made very carefully so that the manufactured parts are not subjected to additional processing. After casting the product and solidifying the metal, the quality of the steel is quite low, since at the boundaries of austenite grains there are small inclusions of carbides, which easily form cracks between the grains and lead to rapid destruction. Turning is possible only with the use of high-speed steels with high heat resistance. That is, the tool, when high temperatures occur at the cutting edge, must maintain high hardness and resist wear.
Features of hardening and welding
To eliminate the low quality of steel after the casting has solidified, it is subjected to a kind of hardening (different from the usual one, which increases the hardness of the metal) at a temperature, depending on the amount of carbon in the alloy, from 900 to 1100 degrees.
- If carbon is 1%, then the temperature should not be lower than 900 degrees.
- If carbon is 1.5% - 1000 degrees.
- When the amount of carbon is 1.6%, the heating temperature is above 1050 degrees.
Heating should be very slow, no more than 150 degrees per hour, followed by holding depending on the size of the casting and final cooling with water.
With a casting thickness of 30 mm, 4 hours of exposure will be required, and with a casting thickness of 125 mm - a day. This treatment completely removes hardening, transforms the metal into austenite, leveling its structure. Accordingly, the hardness of steel after quenching is low, and the toughness is high.
When welding this type of steel, it is necessary to take into account its features. In the heat-affected zone and in the deposited metal, due to the change during heating from the austenitic structure of the metal to martensitic, there is a high probability of cold cracks due to low, 4-6 times less, compared to other types of steels, thermal conductivity and increased by 1 .9 times the coefficient of thermal expansion. There is also a possibility of hot cracks appearing, since the casting shrinkage of Hadfield steel is more than one and a half times greater than that of any low-carbon steel. Therefore, it is recommended to carry out welding work in running water, or, in extreme cases, with subsequent cooling of the seam.
Standards
| Name | Code | Standards |
| Castings with special properties (iron and steel) | B83 | GOST 21357-87, GOST 2176-77, KSt 81-033:2009, TU 48-22-98-83, TU 14-1-563-73, TU 14-1-641-73, TU 4112-78269737-001 -2005 |
| Rails. Overlays. Linings. Crutches | B42 | GOST 7370-98, GOST 28370-89, TU 32-TsP-671-93 |
| Steel castings | B82 | GOST 977-88, OST 24.920.01-80, KSt 81-038:2009, TU 108.11.549-87, TU 14-1-4788-90 |