Argon is one of the most common chemical elements present in the atmosphere of planet Earth, ranking third after nitrogen and oxygen. At the same time, obtaining argon is a responsible and complex process from a technological point of view, which only real professionals can handle. Its peculiarity lies in the fact that in industry this inert gas is obtained by separating air into nitrogen and O2.
But there is also a certain difficulty - the boiling point of this substance is between the boiling temperatures of these two gases. To be more precise, it differs by less than 3 degrees from the value at which O2 boils. Because of this, it is incredibly difficult to separate the two fractions using the rectification method. After all, Ar will simply be distributed among other substances-components of the air, joining oxygen in most cases.
But the widespread distribution of Ar and its demand in a variety of fields of activity have forced humanity to develop more and more new methods and technological methods to make the process of producing argon simpler, more convenient and more effective. Moreover, many of them are associated with the production of industrial gases that have undergone deep purification (you can read our separate material on the topic of especially pure gaseous substances).
History of discovery
The background to the discovery of Ar began in 1785. An outstanding scientist and naturalist from Great Britain, Henry Cavendish, studied the composition of air. He subjected nitrogen to oxidation and weighed the resulting oxides. At the end of the experiment, gas remained in the vessel. Cavendish determined its volume to be 0.8% of the initial volume of air.
The scientist was unable to determine the composition of this gas. A century later, Sirs John Rayleigh and William Ramsay returned to the problem. During their experiments, they discovered that nitrogen released from the air has a higher density than nitrogen obtained during the decomposition reaction of ammonium nitrite.
in 1884 they managed to isolate a certain gas from the air that was denser than nitrogen. This substance had a monatomic molecular structure and was extremely inert - i.e. did not react with other substances.
At a meeting of the Royal Society, the new gas was given the name “argon”, which translated from ancient Greek meant “calm, lazy”
Advantages and disadvantages of welding in argon environment
Argon welding has both pros and cons that must be taken into account when choosing this method. Its advantages are:
- No deformation of workpieces due to high temperature, since it is not necessary to significantly heat the edges of the parts.
- Argon is an inert gas, which means it is heavier than air. This means that oxygen will not be able to penetrate the weld pool.
- The arc has high thermal power, welding occurs at high speed and good quality, if the master is sufficiently qualified.
- Despite some features of argon welding, this process is simple and easy to learn.
- Argon welding can be used to join metals that cannot be joined in any other way.
The disadvantage of argon welding is that it cannot be carried out outdoors in the presence of strong winds. Argon is carried by the wind and therefore does not protect the seam well, so the latter may be of poor quality. In enclosed spaces, welding should be carried out only using forced ventilation. In addition, if it is necessary to use a high-ampere welding arc, then you should decide in advance how the weld will be cooled during the work.
Argon in nature
Due to its almost complete inertness, Ar is present in the natural environment exclusively in unbound form. Its percentage in different parts of the Earth is approximately:
- earth's crust - 0.00012%;
- sea water - 0.00045%;
- atmosphere - 0.926%.
The proportion of Ar in the air is higher than the total proportion of all other inert gases. The main source for its production is our atmosphere.
Content of gases in the atmosphere
Argon is also contained in the Earth's crust in the form of the radioactive isotope Argon-40 and appears during the decay reaction of Potassium isotopes.
Modern science, along with other inert gaseous elements, classifies Ar into group VIII of the periodic table.
Types of equipment for argon arc welding
There are several types of argon welding, which depend on the level of mechanization of the process:
- manual;
- mechanized;
- automated;
- robotic.
Each welding method requires certain equipment, and accordingly, the cost of the work varies.
In the manual welding method, non-consumable tungsten electrodes are used, the wire is fed by the master himself, and the torch for argon arc welding moves.
The mechanized method is characterized by the supply of filler rods in automatic mode, while the master himself holds the torch.
Automated welding is completely under operator control - wire feeding and torch movement occur automatically.
The robotic process also eliminates the presence of an operator.
How is argon produced?
Due to the industrially significant content of argon in the air, it is obtained as an additional product of the cryogenic distillation of O2 and N2.
The technology is based on the fact that the boiling (or liquefaction) point of Ar lies between the temperatures of N2 and O2.
Before the process begins, the air is thoroughly cleaned of dust in multi-stage filters, dried from water vapor, and then compressed by powerful compressors until it turns into a liquid state. The liquid is distilled in a distillation column to separate it into its individual substances.
Installation for argon production
Nitrogen is the first to evaporate at -195 °C; its vapors are collected on the appropriate rectifier plate and discharged into a separate tank. The next highest (and at a boiling point of -185 °C) is the argon fraction, containing 12% Ar, less than half a percent nitrogen and oxygen. It is fed into the next distillation column, in which the percentage of Ar is brought to 85, the remainder being oxygen with traces of nitrogen. This substance is called raw argon, the starting material for producing purified gas.
Several methods are used in industry to purify raw argon from impurities.
Hydrogen added to the raw material is oxidized by a catalyst and heated to 500 °C, thus removing oxygen from the mixture. The water vapor formed on the catalyst is removed using a moisture separator. The gas is then dried. Argon with the nitrogen remaining in it is rectified again.
Alternative methods for obtaining Ar are also used. During the synthesis of ammonia from nitrogen and hydrogen in chemical reactors, Ar is obtained as a by-product. The technological component of this synthesis - purge gas - contains up to 20% Ar. The calmest element is extracted from this gas. The production cost, which consists mainly of the costs of cooling and heating the components, is divided between ammonia and argon, and is significantly lower.
The quality of the gas obtained by any method is determined by the technology for purifying it from small amounts of residual N2, O2, water vapor and H2.
A device that receives argon ion beams
Where is argon used?
Argon has become widespread in industry. The inert properties of this gas are especially in demand in various production processes where it is necessary to displace one of the most active elements - oxygen. The use of argon is very cheap compared to other inert volatile substances, so the gas is indispensable when a protective environment is required when welding metals, as well as displacing moisture and oxygen in containers where food products are stored.
Filling the bulbs of incandescent lamps with inert gas can significantly increase the operating life of the lighting device. In addition to increased service life, such elements have greater brightness. Inert gas is also used in the production of fluorescent lamps. The use of argon makes it easier to start an electric arc discharge, as well as significantly increase the service life of the electrodes.
When making double-glazed windows, the cavities between the glasses are filled with inert gas, which can significantly improve the thermal insulation properties. Considering the fact that argon is absolutely transparent, its use is not limited in any way even in the manufacture of multilayer structures.
The inert gas argon is also used in plasma cutting systems for metals. The advantage of using this gas is that it does not require too high a voltage to initiate an arc, so such installations can be of very simple design. Plasma generation using argon produces minimal harmful gases during cutting, making this method ideal for hand-held devices.
Due to the ability to form plasma at a relatively low voltage, this noble gas is used in medicine for argon coagulation. This method is successfully used to remove tumors, as well as to stop bleeding.
Argon is also used in the chemical industry. Due to the lack of interaction with other elements, this gas is used to obtain ultrapure substances, as well as for their analysis. In the metallurgical industry, noble gas allows the processing of metals such as titanium, tantalum, niobium, beryllium, zirconium, etc. In addition, the gas is used to mix molten substances and reduce the oxidation of chromium in the production of chrome steel.
General characteristics of Ar
Ar belongs to the group of inert gases. The charge of its nucleus is 18; the element is located under the same number in the periodic table.
Of all the members of group VIIIA, it is the most commonly found in nature. The volume fraction of Ar in the atmosphere is 0.93%, the mass fraction is 1.28%. The element is a colorless, tasteless and odorless gas. Chemically inactive - argon does not react and practically does not combine with any elements or substances, with the exception of CU(Ar)O and argon hydrofluoride.
Very poorly soluble in water, slightly greater solubility is observed when interacting with organic solvents.
Properties of argon (table): temperature, density, pressure, etc.
| Name | Argon |
| Latin name | Argon |
| English name | Argon |
| Symbol | Ar |
| Atomic number (number in table) | 18 |
| Type | Non-metal |
| Group | Inert (noble) gas |
| Open | William Ramsay, John William Strett (Lord Rayleigh), UK, 1894 |
| Opening year | 1894 |
| Appearance, etc. | An inert gas without color, taste or smell |
| Origin | Natural material |
| Content in the atmosphere and air (by mass) | 1,292 % |
| Content in the earth's crust (by mass) | 0,00015 % |
| Content in seas and oceans (by mass) | 0,000045 % |
| Content in the Universe and space (by mass) | 0,02 % |
| Abundance in the Sun (by mass) | 0,007 % |
| Atomic mass (molar mass) | 39.948(1) a. e.m. (g/mol) |
| Electronic configuration | 1s2 2s2 2p6 3s2 3p6 |
| Electronic shell | K2 L8 M8 N0 O0 P0 Q0 R0 |
| Atomic radius (calculated) | 71 pm |
| Covalent radius* | 106 pm |
| Van der Waals radius | 188 pm |
| Electrons, Protons, Neutrons | 18 electrons, 18 protons, 22 neutrons |
| Family (block) | p-family element |
| Period in the periodic table | 3 |
| Group on the periodic table | 18th group (according to the old classification - the main subgroup of the 8th group) |
| Oxidation states | 0 |
| Valence | 0 |
| Electronegativity | 4.3 (Pauling scale) |
| Ionization energy (first electron) | 1520.57 kJ/mol (15.7596117(5) eV) |
| Electrode potential | 0 |
| Electron affinity energy of an atom | 0 kJ/mol |
| Density* | 0.001784 g/cm3 (at 0 °C and other standard conditions, the state of matter is gas), 1.3954 g/cm3 (at a boiling point of -185.848 °C and other standard conditions, the state of matter is liquid), 1.65 g/cm3 (at -233 °C and other standard conditions, state of matter – solid) |
| Melting temperature* | -189.34 °C (83.81 K, -308.81 °F) |
| Boiling temperature* | -185.848 °C (87.302 K, -302.526 °F) |
| Specific heat of fusion (enthalpy of fusion ΔHpl)* | 1.18 kJ/mol |
| Specific heat of evaporation (enthalpy of boiling ΔHboiling)* | 6.53 kJ/mol |
| Molar heat capacity* | 20.85 J/(K mol) |
| Molar volume | 24.2 cm³/mol |
| Thermal conductivity | 17.72 10-3 W/(m K) (at standard conditions), 0.0164 W/(m K) (at 300 K) |
| Lattice structure | Cubic face centered |
| Lattice parameters | 5.260 Å |
| Debye temperature | 85 K |
| Name of space symmetry group | Fm_3m |
| Symmetry space group number | 225 |
| CAS number | 7440-37-1 |
Note:
206* The covalent radius of argon according to [1] is 106±10 pm.
401* The density of argon according to [3] and [4] [Russia] is 0.0017839 g/cm3 (at 0 °C /20 °C and other standard conditions, the state of the substance is gas).
402* The melting point of argon according to [3] and [4] is -189.35 °C (83.8 K, -308.83 °F) and -189.6 °C (83.55 K, -309.28 °F) respectively.
403* The boiling point of argon according to [3] and [4] is -185.85 °C (87.3 K, -302.53 °F) and -185.9 °C (87.25 K, -302.62 °F) respectively.
407* The specific heat of fusion (enthalpy of fusion ΔHmelt) of argon according to [3] and [4] is 7.05 kJ/mol and 1.19 kJ/mol, respectively.
408* The specific heat of evaporation (enthalpy of boiling ΔHboil) of argon according to [3] and [4] is 6.45 kJ/mol and 6.51 kJ/mol, respectively.
410* The molar heat capacity of argon [3] is 20.79 J/(K mol).
Types of argon
When talking about types or varieties of Ar, we must understand that these are the same chemical substance. Types differ in the degree of purification from impurities.
- Top grade. Ar content is not less than 99.99%. This grade of especially high purity is used for critical welding work, such as welding materials that are chemically active in a heated state: some non-ferrous alloys, primarily titanium, stainless steel, etc. It is also used for welding highly loaded structural steel products.
- First grade. Ar content not less than 99.98%, used for welding aluminum-based alloys with other metals and alloys, for less active non-ferrous metals.
- Second grade. Ar content not less than 99.95%. Used for welding parts made of heat-resistant steel alloys, aluminum and structural steels. The use of pure Ar in these cases is undesirable, since it leads to increased porosity of the weld material and does not protect the weld pool from high humidity and other contaminants. To avoid the occurrence of such a defect, carbon dioxide and oxygen are added to the mixture of protective gases, which bind the hydrogen and other impurities released during welding. The slags formed during these reactions float to the surface of the weld pool and, after solidification, are removed along with the scale.
Argon production
Industrial argon gas is produced as a by-product of a manufacturing process during which oxygen is separated from nitrogen. For this purpose, special chambers are used using air separation devices with double rectification. Argon is more volatile than oxygen and less volatile than nitrogen. Therefore, during the separation of air into oxygen and nitrogen, argon remains in the middle fraction. From the midpoint of the upper column of the apparatus, argon is directed into special chambers for compression and storage.
During primary selection, the mass fraction of argon in the selected fraction is negligible, only about five percent. This is the so-called raw argon. After subsequent condensation and purification, it is possible to obtain pure argon with a mass fraction of its content in the fraction of about 99.99 percent. A method of extracting argon during the disposal of ammonia production waste is also practiced. In this case, argon is obtained from the nitrogen remaining after binding it with hydrogen molecules.
Physical and chemical properties
The properties of argon are typical of a member of group VIII.
At ordinary temperatures, Ar is in a gaseous state. The molecule includes a single atom, the chemical formula is very simple: Ar. The boiling point is very low: -185.8 °C at atmospheric pressure.
Solubility in water is low - only 3.29 ml per 100 ml of liquid
The density of argon under normal conditions is 1.78 kg/m3. The molar heat capacity of the gas is 20.7 J/Kmol.
Characteristics of argon and other inert gases
The gas is almost completely inert. To date, scientists have managed to obtain only two of its compounds - CU(Ar)O and argon hydrofluoride. The compounds exist only at ultra-low temperatures. It is assumed that Ar may be part of excimer-type molecules that are unstable in the normal state. Such molecules can only exist in an excited state, for example, during a high-intensity electrical discharge. Such compounds are possible with mercury, oxygen and fluorine.
Electronegativity on the Pauling scale is 4.3.
Both the oxidation state and the electrode potential have a zero value, which is typical for an inert gas.
The ionic radius is 154, the covalence radius is 106 PM. Ionization threshold - 1519 kJ/mol
Atomic and molecular mass
Such important parameters as atomic and molecular masses show how much the mass of a molecule of a substance and the mass of its atom, respectively, exceed a value equal to one twelfth of the mass of a hydrogen atom.
Due to the fact that the Ar molecule consists of a single atom, the molecular and atomic mass of argon are identical and amount to 39.984.
Argon structure and properties
Isotopes
Under natural conditions, Ar occurs as three stable isotopes
- 36Ar – the percentage of this isotope is 0.337% in the nucleus of 18 protons and 18 neutrons;
- 38Ar - its share is only 0.063%, there are 18 protons and 20 neutrons in the nucleus;
- 40Ar is the most common, its share is 99.6%, the nucleus also has 18 protons, but already 22 neutrons.
It was possible to artificially obtain isotopes with a mass index from 32 to 55, the most stable of which was 39Ar, whose half-life is 268 years.
The large percentage of 40Ar among the isotopes found in nature is caused by its constant formation during the decay reaction of the potassium-40 isotope. Per 1000 kg of potassium during such reactions no more than 3100 40Ar atoms are formed per year. But, since these reactions take place continuously over hundreds of millions of years, the isotope has accumulated in nature in significant volumes.
The dominance of the heavy isotope in nature determines the fact that the atomic weight of Ar exceeds the atomic weight of potassium, which is located next to it in the table. When the Periodic Table was created, there was no such contradiction, since argon was discovered and its properties were studied much later, in the first decade of the 20th century. Ar was initially placed in the first group of the table; the eighth group was allocated later.
Ions
Like other noble gases (such as He and Ne), Ar is susceptible to ionization. When atoms are excited and given high energies, molecular Ar2+ ions appear.
Molecule and atom
For inert gases, these concepts are identical, since these elements do not want to enter into a chemical bond even with their own kind. The molecule includes one atom, the chemical formula of the gas does not differ from the designation of the element: Ar.
Molar mass
The molar mass of argon is 39.95 g/mol.
There are several methods for calculating it:
- Using the relative atomic mass M and the proportionality coefficient k, expressing the relationship between the relative mass and the molar mass. This coefficient is a universal constant and is equal for all elements. Molar mass M is expressed as the product of the proportionality coefficient and the relative mass.
- Using molar volume. You will need to find the volume occupied by a certain mass of gas under normal conditions, then calculate the mass of 22.4 liters of the substance under the same conditions.
- Using the Mendeleev-Clapeyron equation simulating an ideal gas.
pV = mRT / M,
Having carried out the transformations, we obtain the expression for the molar mass:
M=mRT/pV
Where
- p – pressure in pascals,
- V – volume in cubic meters
- m – mass in grams,
- T - temperature in Kelvin,
- R is a constant whose value is 8.314 J/(mol×K).
What is argon?
It is a monatomic inert gas that does not have any odor, color or taste. It is one of the main components of air and is also found in large quantities in the atmosphere of our planet. Its boiling point is minus 185.9 degrees Celsius, which is very close to the boiling point of O2. It should be noted that, being in the air in high concentrations, it can be dangerous for humans, as it displaces oxygen and leads to symptoms of oxygen starvation.
According to state standards (GOST), gaseous Ar must be stored and transported in gas cylinders specially designed for this purpose, made using a steel alloy. The pure substance must be in containers that are appropriately colored and marked - a gray cylinder with a green inscription: “Pure argon”.
Application area
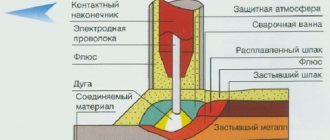
Argon is most widely used for welding. It is used to create a protective atmosphere around the weld pool, displacing O2 and N2 contained in the atmosphere from the working area. This is especially important for welding non-ferrous metals, many of which, for example, Ti, are characterized by high chemical activity when heated. Inert gas is also indispensable for permanent connections of stainless and high-alloy alloys.
It is also widely used in the installation of highly loaded building structures, such as frames of high-rise buildings, bridge trusses and many others. Here its use ensures high quality, uniformity and durability of critical connections. In the construction industry, argon welding dominates among other methods.
Argon welding
Argon arc welding
Argon welding is no less widely used in mechanical engineering, primarily in the chemical and food industries. The seams are durable and reliable, even when exposed to aggressive environments.
The oil and gas industries also use argon welding in the installation of pipelines, gas pumping stations and oil refineries.
The method is also used in the nuclear industry, transport engineering and the aerospace industry.
In households, argon welding is not so widespread. This is explained:
- high cost of equipment and consumables;
- the need for sufficient qualification of the welder;
- lower loads experienced by home structures;
- lower requirements for the strength and durability of welded joints.
If a household has an occasional need for such welding work, then it is cheaper, faster and more reliable to invite a specialist welder.
Double-glazed window with argon
The principle of operation of a double-glazed window with argon
A characteristic property of Ar is its higher density compared to air. Therefore, the maximum efficiency of argon welding is achieved in the lower welding position. In this case, the inert substance spreads over the surface of the part and forms a protective cloud of considerable extent, allowing welding to be carried out both with high currents and at high speed. When welding in an inclined and upper position, it is necessary to take into account the “falling through” of argon through the air. To compensate for this phenomenon, either increase the gas supply or carry out work in a sealed room filled with inert gas. In both cases, the cost of work increases.
Since the ionization potential of Ar is low, its use ensures ideal geometric characteristics of the weld, especially the profile. An excited electric arc in an argon atmosphere is also characterized by high stability of its parameters. On the other hand, a low ionization potential also causes a lower arc ignition and maintenance voltage. This reduces its heat generation and complicates the penetration of thick sheets of metal.
The higher arc temperature in an argon atmosphere significantly increases weld penetration. This allows welding to be carried out in one pass, provided that the parameters of the gap between the workpieces are strictly observed.
When using the TIG welding method, the argon atmosphere protects not only the welding zone, but also the end of the infusible electrode from corrosive influences.
In a number of specific cases, helium is added to the protective gas mixture.
In addition to being used for welding, argon is used:
- As a plasma-forming substance in installations for plasma cutting of metal.
- To create an inert environment in food packaging. It displaces air oxygen and water vapor from bags and containers, which adversely affect the shelf life of products. Products in a protective atmosphere are stored several times longer than in conventional packaging. This method is also used for packaging medical products and drugs, allowing them to be kept in proper sterility and chemical purity.
- As an active agent in fire extinguishing installations. Argon displaces oxygen (or other gas) from the combustion site, stopping it.
- To create a protective environment in technological installations when processing semiconductor devices, creating microcircuits and other electronic components or materials of high purity levels.
- Filler for electric lamps.
- In advertising fluorescent tubes.
Application
Argon Applications:
- in argon lasers
- in incandescent lamps and when filling the internal space of double-glazed windows
- as a protective medium when welding (arc, laser, contact, etc.) both metals and non-metals
- as a plasma generator in plasmatrons for welding and cutting
- in the food industry, argon is registered as a food additive E938, as a propellant and packaging gas
- as a fire extinguishing agent in gas fire extinguishing installations
Biological role
Argon plays no biological role.
Physiological action
Inert gases have a physiological effect, which is manifested in their narcotic effect on the body. The narcotic effect of inhaling argon appears only at barometric pressure above 0.2 MPa. High concentrations of argon in the inhaled air can cause dizziness, nausea, vomiting, loss of consciousness and death from asphyxia (as a result of oxygen starvation).
Mendeleev's periodic table of chemical elements
Classification of chem. elements, establishing the dependence of various properties of elements on the charge of the atomic nucleus. The system is a graphic expression of the periodic law/
Periodic table of elements
| I.A. | IIA | IIIB | IVB | VB | VIB | VIIB | —- | VIIIB | —- | I.B. | IIB | IIIA | IVA | V.A. | VIA | VIIA | VIIIA | |
| Period | ||||||||||||||||||
| 1 | 1H Hydrogen | 2 He Helium | ||||||||||||||||
| 2 | 3 Li Lithium | 4 Be Beryllium | 5 B Boron | 6 C Carbon | 7 N Nitrogen | 8 O Oxygen | 9 F Fluorine | 10 Ne Neon | ||||||||||
| 3 | 11 Na Sodium | 12 Mg Magnesium | 13 Al Aluminum | 14 Si Silicon | 15 P Phosphorus | 16 S Sulfur | 17 Cl Chlorine | 18 Ar Argon | ||||||||||
| 4 | 19 K Potassium | 20 Ca Calcium | 21 Sc Scandium | 22 Ti Titan | 23 V Vanadium | 24 Cr Chrome | 25 Mn Manganese | 26 Fe Iron | 27 Co Cobalt | 28 Ni Nickel | 29 Cu Copper | 30 Zn Zinc | 31 Ga Gallium | 32 Ge Germanium | 33 As Arsenic | 34 Se Selenium | 35 Br Bromine | 36 Kr Krypton |
| 5 | 37 Rb Rubidium | 38 Sr Strontium | 39 Y Yttrium | 40 Zr Zirconium | 41 Nb Niobium | 42 Mo Molybdenum | (43) Tc Technetium | 44 Ru Ruthenium | 45 Rh Rhodium | 46 Pd Palladium | 47 Ag Silver | 48 Cd Cadmium | 49 In Indium | 50 Sn Tin | 51 Sb Antimony | 52 Te Tellurium | 53 I Iodine | 54 Xe Xenon |
| 6 | 55 Cs Cesium | 56 Ba Barium | * | 72 Hf Hafnium | 73 Ta Tantalum | 74 W Tungsten | 75 Re Rhenium | 76 Os Osmium | 77 Ir Iridium | 78 Pt Platinum | 79 Au Gold | 80 Hg Mercury | 81 Tl Thallium | 82 Pb Lead | 83 Bi Bismuth | (84) Po Polonium | (85) At Astatine | 86 Rn Radon |
| 7 | 87 Fr Franc | 88 Ra Radium | ** | (104) Rf Rutherfordium | (105) Db Dubnium | (106) Sg Seaborgium | (107) Bh Borium | (108) Hs Khassiy | (109) Mt Meitnerium | (110) Ds Darmstadtium | (111) Rg X-ray | (112) Cp Copernicium | (113) Uut Ununtriy | (114) Uuq Ununquadium | (115) Uup Ununpentius | (116) Uuh Unungexium | (117) Uus Ununseptius | (118) Uuo Ununoctium |
| 8 | (119) Uue Ununenniy | (120) Ubn Unbinilium | ||||||||||||||||
| Lanthanides* | 57 La Lantan | 58 Ce Cerium | 59 Pr Praseodymium | 60 Nd Neodymium | (61) Pm Promethium | 62 Sm Samaria | 63 Eu Europium | 64 Gd Gadolinium | 65 Tb Terbium | 66 Dy Dysprosium | 67 Ho Golmi | 68 Er Erbium | 69 Tm Thulium | 70 Yb Ytterbium | 71 Lu Lutetium | |||
| Actinoids** | 89 Ac Actinium | 90 Th Thorium | 91 Pa Protactinium | 92U Uranium | (93) Np Neptunium | (94) Pu Plutonium | (95) Am Americium | (96) Cm Curium | (97) Bk Berkelium | (98) Cf Californian | (99) Es Einsteinium | (100) Fm Fermium | (101) Md Mendelevium | (102) No Nobels | (103) Lr Lawrence | |||
Chemical families of periodic table elements
| Alkali metals | Alkaline earth metals | Lanthanides | Actinoids | Transition metals |
| Light metals | Semimetals | Nonmetals | Halogens | Noble gases |
Dependence of argon pressure in a cylinder on temperature
As it heats up, the pressure of the gaseous substance in a closed volume increases. The table shows approximate pressure values in the cylinder depending on the ambient temperature.
| T, °C | P, Megapascal |
| -40 | 10,45 |
| -30 | 11,33 |
| -20 | 12,21 |
| -10 | 12,92 |
| 0 | 13,74 |
| +10 | 14,62 |
| +20 | 15,33 |
| +30 | 16,03 |
It should be borne in mind that the balloon pressure does not change instantly, but as it warms up or cools down.
Areas of application of argon welding
What is argon welding used for? It is necessary in cases where welding seams must be performed flawlessly. It is especially often used to connect difficult-to-weld materials and workpieces with thin walls. This type of welding is in demand in aircraft and rocket manufacturing, and the automotive industry. Through this connection, important components are made from aluminum and its alloys.
Most often, argon arc welding is used when working with aluminum, which is difficult to weld, often cracks, and shrinks severely. In addition, in the molten state, this metal easily oxidizes, becoming covered with a refractory film, which prevents the formation of a seam. And only welding in an argon environment will help to obtain high-quality seams.
Such welding is especially in demand at automobile service stations, where with the help of this joining method the service life of parts is significantly extended.
Why is argon welding used at car service stations? It can be used to repair radiators, various parts of gearboxes, air conditioner pipes and other elements made of aluminum and its alloys. Soldering and plasma spraying, as well as other welding methods, could not be used for such work, since the parts have technical features.
Getting Argon
3.3 Preparation of Argon.
The Earth's atmosphere contains 66 · 1013 tons of argon. This source of argon is inexhaustible, especially since almost all argon sooner or later returns to the atmosphere, since it does not undergo any physical or chemical changes when used. The exception is very small amounts of argon isotopes, which are spent to produce new elements and isotopes in nuclear reactions. Argon is produced as a by-product when air is separated into oxygen and nitrogen. Typically, double rectification air separation devices are used, consisting of a lower high-pressure column (pre-separation), an upper low-pressure column and an intermediate condenser-evaporator. Ultimately, nitrogen is removed from above, and oxygen from the space above the condenser. The volatility of argon is greater than that of oxygen, but less than that of nitrogen. Therefore, the argon fraction is selected at a point located approximately at a third of the height of the upper column and taken to a special column. Composition of the argon fraction: 10...12% argon, up to 0.5% nitrogen, the rest is oxygen. In an “argon” column connected to the main apparatus, argon is produced with an admixture of 3...10% oxygen and 3...5% nitrogen. Next comes the purification of “raw” argon from oxygen (chemically or by adsorption) and from nitrogen (by rectification). Argon up to 99.99% purity is now produced on an industrial scale. Argon is also extracted from ammonia production waste - from the nitrogen remaining after most of it has been bound with hydrogen. Argon is stored and transported in cylinders with a capacity of 40 liters, painted gray with a green stripe and green inscription. The pressure in them is 150 atm. It is more economical to transport liquefied argon, for which Dewar flasks and special tanks are used. Artificial radioisotopes of argon were obtained by irradiating some stable and radioactive isotopes (37Cl, 36Ar, 40Ar, 40Ca) with protons and deuterons, as well as by irradiating products formed in nuclear reactors during the decay of uranium with neutrons. The isotopes 37Аr and 41Аr are used as radioactive tracers: the first - in medicine and pharmacology, the second - in the study of gas flows, the effectiveness of ventilation and in various scientific research. But, of course, these are not the most important uses of argon.
3.4 Physiological effect of inert gases.
It was natural to expect that such chemically inert substances as inert gases should not affect living organisms. But that's not true. Inhalation of higher inert gases (of course, mixed with oxygen) leads a person to a state similar to intoxication with alcohol. The narcotic effect of inert gases is caused by dissolution in nerve tissues. The higher the atomic weight of an inert gas, the greater its solubility and the stronger its narcotic effect.
Now about the effect of argon on a living organism. When inhaling a mixture of 69% Ar, 11% nitrogen and 20% oxygen under a pressure of 4 atm, narcosis phenomena occur, which are much more pronounced than when inhaling air under the same pressure. The anesthesia disappears instantly after stopping the argon supply. The reason is the non-polarity of argon molecules, while increased pressure increases the solubility of argon in nerve tissues. Biologists have found that argon promotes plant growth. Even in an atmosphere of pure argon, the seeds of rice, corn, cucumbers and rye sprouted. Onions, carrots and lettuce grow well in an atmosphere consisting of 98% argon and only 2% oxygen.
IV Application of inert gases.
Helium is an important source of low temperatures. At the temperature of liquid helium, there is virtually no thermal movement of atoms and free electrons in solids, which makes it possible to study many new phenomena, such as superconductivity in the solid state.
Helium gas is used as a light gas to fill balloons. Because it is non-flammable, it is added to hydrogen to fill the airship's shell.
Since helium is less soluble in the blood than nitrogen, large quantities of helium are used in breathing mixtures for work under pressure, for example during sea diving, when creating underwater tunnels and structures. When using helium, decompression (release of dissolved gas from the blood) is less painful for a diver, decompression sickness is less likely, and the phenomenon of nitrogen narcosis, a constant and dangerous companion to a diver’s work, is eliminated. He–O2 mixtures are used, due to their low viscosity, to relieve asthma attacks and for various respiratory diseases.
Helium is used as an inert medium for arc welding, especially magnesium and its alloys, in the production of Si, Ge, Ti and Zr, for cooling nuclear reactors.
Other uses of helium are for gas lubrication of bearings, in neutron counters (helium-3), gas thermometers, X-ray spectroscopy, food storage, and high voltage switches. Mixed with other noble gases, helium is used in outdoor neon advertising (in gas discharge tubes). Liquid helium is beneficial for cooling magnetic superconductors, particle accelerators and other devices. An unusual application of helium as a refrigerant is the process of continuously mixing 3He and 4He to create and maintain temperatures below 0.005 K
The areas of application of xenon are varied and sometimes unexpected. Man takes advantage of both its inertness and its wonderful ability to react with fluorine. In lighting technology, high-pressure xenon lamps have gained recognition. In such lamps, an arc discharge shines in xenon, which is under a pressure of several tens of atmospheres. The light in xenon lamps appears immediately after switching on, it is bright and has a continuous spectrum - from ultraviolet to near-infrared. Doctors also use xenon for fluoroscopic examinations of the brain. Like barite porridge, which is used for intestinal candling, xenon strongly absorbs x-rays and helps to find lesions. However, it is completely harmless. The active isotope of element No. 54, xenon - 133, is used in studying the functional activity of the lungs and heart.
By blowing argon through liquid steel, gas inclusions are removed from it. This improves the properties of the metal.
Electric arc welding in an argon environment is increasingly being used. In an argon jet it is possible to weld thin-walled products and metals that were previously considered difficult to weld. It would not be an exaggeration to say that the electric arc in an argon atmosphere revolutionized the technology of cutting metals. The process was much faster, and it became possible to cut thick sheets of the most refractory metals. Argon blown along the arc column (mixed with hydrogen) protects the cut edges and the tungsten electrode from the formation of oxide, nitride and other films. At the same time, it compresses and concentrates the arc on a small surface, causing the temperature in the cutting zone to reach 4000-6000 ° C. In addition, this gas jet blows out the cutting products. When welding in an argon jet, there is no need for fluxes and electrode coatings, and therefore, there is no need to clean the seam from slag and flux residues.
Neon and argon are used as fillers in neon lamps and daylight lamps. Krypton is used to fill ordinary lamps in order to reduce evaporation and increase the brightness of the tungsten filament. High-pressure quartz lamps, which are the most powerful light sources, are filled with xenon. Helium and argon are used in gas lasers.
List of used literature
1. Petrov M.M., Mikhilev L.A., Kukushkin Yu.N. "Inorganic chemistry"
2. Guzey L.S. Lectures on general chemistry”
3. Akhmetov N.S. “General and inorganic chemistry”
4. Nekrasov B.V. “Textbook of General Chemistry”
5. Glinka N.L. "General chemistry
6. Khodakov Yu.V. “General and inorganic chemistry”
... transformations of isolated molecules. But this is a topic for a separate article. For our history, it is important that such matrix isolation, unexpectedly for everyone, led to a completely new field of inert gas chemistry. And this happened as a result of one meeting at an international conference on matrix isolation in the USA, which took place in 1995. It was then that the scientific world first learned about the existence of new...
... in water (3.29 cm3 in 100 g of water at 20°C). Argon dissolves even better in many organic liquids. But it is practically insoluble in metals and does not diffuse through them. Like all inert gases, argon is diamagnetic. This means that its magnetic susceptibility is negative, it has greater resistance to magnetic lines of force than emptiness. This is a property of argon (like many others) ...
... welding is low productivity when using the manual version. The use of automatic welding is not always possible for short and differently oriented seams. 5. Technology of manual welding with a non-consumable electrode in inert gases Argon welding can be manual, when the torch and filler rod are in the hands of the welder, and automatic, when the torch and ...
... SEU. Justification of the relevance of the ongoing development. Analysis of specialized literature, patent search. Calculation of elements of the inert gas system. Create a SIG diagram. Draw a general view drawing of the scrubber for SIG. Conclusions and proposals based on the development results. DEVELOPMENT OF INSTALLATION TECHNOLOGY (MANUFACTURE, ASSEMBLY OR TESTING) OF A SPP ELEMENT. Development of technological…
How to make argon welding with your own hands from an inverter
Argon welding is an indispensable method with which you can create permanent joints of products made of non-ferrous metals, titanium, stainless steel and other alloys. In addition, this type of welding is characterized by good seam quality and high productivity. The universal capabilities of argon welding also attract home craftsmen. But this equipment is expensive and is practically not purchased for home use. Therefore, more and more craftsmen are starting to think about making an argon welding unit with their own hands.
Technology and application of argon welding
Argon welding is a bit like ordinary arc welding, but it uses a shielding gas, argon, to protect the weld pool. This inert gas has a number of properties unique to it.
- Since argon is 38% heavier than air, it penetrates well into the weld pool and protects it from gases in the atmosphere. Thanks to this, the welding seam is obtained without the formation of an oxide film, which improves the quality of the connection.
- Argon is present in the air, so it is a byproduct of oxygen and nitrogen produced from the atmosphere, and is the least expensive of the shielding gases for welding.
The welding process in argon occurs according to the following principle. Literally 1 second before the arc ignites, argon is supplied to the burner. The welder brings the electrode to the part prepared for connection and presses the power button. But since high ionization is required to ignite an arc in a protective gas environment, an oscillator comes into play.
An oscillator is a device that produces high-frequency and high-voltage pulses that can ionize a gas and ignite an arc between the electrode and the workpiece.
After ignition of the arc, filler wire is supplied to the joint of the parts manually or automatically. Parts are welded by melting the additive, the metal of which falls on the molten edges of the workpieces being joined.
Traditionally, argon welding refers to the joining of metals using a non-consumable tungsten electrode that creates an arc and an additive in the form of a metal rod or wire. This type of welding has the international designation “TIG”.
Argon welding is used in the following areas.
- Frame construction. Welded seams can withstand constant loads.
- Joining of pipes of both steel and non-ferrous metals, including pipes of various alloys.
- Connection of dissimilar metals.
- Splicing of almost any metals together: titanium, copper, aluminum, stainless steel, bronze, brass, cast iron, etc. This is especially important for the automotive industry.
- Manufacturing of decorative and jewelry items.
Elements for assembling a homemade apparatus
To assemble equipment for argon welding, you will need the following items:
- DC or inverter type welding machine;
- oscillator;
- inverter protection unit;
- burner;
- argon cylinder;
- gas reducer;
- gas hose;
- welding cables.
Current source
As a current source for TIG welding, you can take a regular welding transformer and adapt a diode bridge at its output to rectify the current. You can also use a welding straightener. But for both types of devices, you will also need to add an oscillator, which will facilitate contactless ignition of the arc.
On the Internet you can read that the easiest way to do argon welding is from an inverter. But there are several nuances here. There are inverters that already have built-in TIG welding capabilities. In this case, it is enough to connect a hose with a torch for argon welding to the device, connect the hose to an argon cylinder, and the unit is ready for work. But first you need to switch it to TIG mode and set the required current.
It should be noted that such inverters already have a built-in oscillator and the necessary protection.
Inverters without a built-in TIG welding function cannot be used for this purpose. Even if you connect an external oscillator to it, the inverter will simply burn out. To prevent this from happening, you will need a small modification of the inverter, which consists of adding a protection unit to its circuit. This block can be assembled together with the oscillator on one board and placed in a separate case. You will get a small attachment for the inverter.
Oscillator and protection unit
As mentioned above, a welding inverter will require a special attachment for TIG welding. You can assemble it with your own hands according to the diagram provided below.
This circuit includes a protection block (located on the left) and an oscillator. The latter can be purchased in China or assembled yourself. You can find out how the above circuit is assembled by watching this video.
Burner
For argon welding, a special torch is used, consisting of a ceramic nozzle and a tungsten electrode holder.
Also on the burner there is a start button and a gas supply valve. The burner can be assembled from components, which are sufficient on Chinese websites, or you can buy a ready-made (assembled) one there.
Argon cylinder
For safety reasons, it is customary to paint all gas cylinders in different colors and put inscriptions on them in different colors. Below is a picture that shows all types of gas cylinders with markings and colors corresponding to their contents.
As can be seen from the figure, black (with a white stripe) or gray (with a green stripe and inscription) cylinders are used for argon. For TIG welding, purified argon is used. Therefore, you will need to purchase a gray cylinder with a green inscription “Pure Argon”.
Advice! For professional use, cylinders with a capacity of about 50 liters and a large weight are used. But for domestic use, a 10-liter cylinder will be sufficient, which can be moved independently.
Gearbox
Since the gas in the cylinder is under high pressure, a reducer is required to supply it to the burner. This device shows the pressure in the cylinder and allows you to adjust the gas flow rate through the hose leading to the burner.
The reducer must be selected strictly for a specific gas, that is, in this case, for argon. Usually the device has the same color as the gas cylinder.
Hose and welding cables
If you assemble a hose for argon welding yourself, it will turn out to be thick and difficult to bend, since you need to place an electric cable and a gas hose in it. In addition, you will need to separately purchase connectors for connecting to the torch and to the inverter (if you use an inverter with TIG welding capabilities). A ready-made sleeve for argon welding can be purchased in the same place as the torch.
Algorithm for assembling a welding machine
Assembling equipment for argon welding from an inverter is quite simple.
- Connect the protective unit with the oscillator to the inverter according to the diagram above.
- The ground cable must be connected to the oscillator terminal with the “+” sign. The cable that goes to the burner is connected to the terminal with the “-” sign. For aluminum welding, the cables are connected in reverse.
- Connect the burner to the sleeve with the cable and gas hose.
- Screw the reducer to the argon cylinder.
- The gas hose must be connected to a reducer mounted on an argon cylinder.
- Connect the inverter to a 220 V network, and the oscillator to a 6 V power supply.
After this, the DIY TIG welding machine will be ready for use. But first it must be configured correctly.
Setting up finished equipment
A homemade argon welding installation requires the following settings.
- Sharpen the tungsten electrode on a sharpener until it looks like a needle. This is done so that the arc is concentrated at the end of the needle and does not “walk” in different directions.
- Take a torch and install a tungsten electrode into it. The diameter of the electrode must correspond to the collet in which it is fixed.
- Open the valve on the burner and adjust the required argon flow rate using the reducer (a flow rate of 12-15 l/min will be sufficient), then close the valve on the burner again.
- Turn on the oscillator and bring the torch with the electrode to the metal to which the ground cable is connected.
- When you press the power button, an arc should appear between the metal and the electrode at a distance of about 0.5 mm.
- Turn on the gas supply and press the button again. In this case, the arc should be ignited at a distance of 10 mm or more.
After carrying out the simple settings described above, we can say that the device with the TIG function is completely ready for work.
Safety precautions when arc welding in argon
When using liquefied gases in work, it is necessary to strictly follow safety regulations. Let us name its main requirements when using argon:
- Welding should not be carried out near flammable substances;
- it is necessary to remove all foreign objects;
- Only certified materials and serviceable equipment should be used;
- before starting work, you must undergo preliminary instruction and gain basic theoretical knowledge;
- During work, be sure to use a mask or safety glasses, preferably “chameleon” glasses.
Despite the fact that argon is quite harmless to the human body, it is better not to inhale it, since it is lighter than oxygen, and therefore it simply pushes it out. Once argon enters the lungs, a person will begin to suffocate. Welding should be carried out in a room with good natural ventilation, otherwise, to instantly remove combustion products, care must be taken to ensure high-quality constant ventilation.