01/19/2022 Author: VT-METALL
From this material you will learn
:
- Differences between alloy steel and carbon steel
- Alloying additives for steel
- Types of Alloy Steel
- Marking of alloy steels
- Applications of alloy steel
An alloy containing less than 45% iron is called steel. In addition to iron, the usual one includes carbon and various impurities. Alloy steel contains additional elements, so-called alloying elements. They are necessary to give the material different properties.
Depending on these additives, alloy steel acquires characteristics that contribute to its wider use. Due to alloying elements, it becomes resistant to the external environment, ductility and strength increase, and qualities that are required to solve certain problems appear. Our article will help you understand the types and grades of alloy steel, as well as its purpose.
Differences between alloy steel and carbon steel
Alloy steel, in addition to the usual impurities, contains additional substances that allow it to meet certain chemical and physical requirements.
In other words, we are talking about carbon steels to which alloying components have been added. There are different degrees of alloying, but even a small content of such elements significantly increases the quality characteristics of the metal.
What steel is considered alloyed? The difference between alloyed and unalloyed metal is the chemical composition. In the first, in addition to standard iron and carbon, there are many additional components that change properties. Whereas in carbon or classic steel there are traces of random impurities, they are not able to greatly affect its characteristics.
VT-metall offers services:
Alloy steel also differs from carbon steel in such features as
:
- resistance to rust and aggressive environments;
- the formation of sparks when the metal is brought to the grinding wheel;
- low load-bearing capacity;
- significant production cost.
Alloying is carried out using two methods:
- Metallurgical
. This is the basic approach in which the necessary components are introduced into the hot metal. Next, the production sets parameters under which chemical reactions proceed in an accelerated mode. - Additional
. In this case, additives are applied in the form of a surface layer, due to which gradual mutual penetration of elements occurs.
High alloy steels
High-alloy steels have a higher content of alloying elements - Cr and Ni (usually not lower than 16% and 7%, respectively). They give such metals the appropriate structure and necessary properties. Highly alloyed steels, compared to less alloyed steels, have high cold resistance, corrosion resistance, heat resistance and heat resistance. Despite the high properties of these steels, their main service purpose is determined by the appropriate selection of alloying composition. Accordingly, they can be divided into three groups: heat-resistant, heat-resistant and corrosion-resistant.
After appropriate heat treatment, high-alloy steels have high strength and plastic properties. Unlike carbon materials, when hardened, these materials acquire increased plastic properties.
The structures of high-alloy steels are very diverse and depend mainly on their chemical composition, that is, on the content of the main elements: chromium (ferritizer) and nickel (austenitizer). The structure is also affected by the content of other alloying elements, ferritizers (Mo, Ti, Si, Al, W, V) and austenizers (Co, Cu, C, B).
Alloying additives for steel
Chemical elements belonging to different groups of the periodic table are added to alloy steels.
Alloying metals in the Russian marking of alloy steels are designated using the Cyrillic alphabet. With their help, the qualities of the material are changed:
- Nickel (N)
. An increase in heat capacity, viscosity, and ductility, with a parallel decrease in fragility, which simplifies the processing of metal by pressure. - Chrome (X)
. Increased hardness and impact resistance. The additive provides good protection against rust - it is for this reason that there is always a lot of chromium in stainless steel. - Niobium (B)
. Increased resistance to acids. - Cobalt (K)
. Improvement of indicators such as resistance to shock and high temperatures. - Copper (D)
. Increasing the strength of alloy steel, however, when using this alloying element, the level of viscosity is slightly reduced. This component is usually added to make construction steel. - Titanium (T) and zirconium (Z)
. Reducing the level of grain size, since these metals provide a homogeneous structure, reduces the likelihood of cracking. - Tungsten (B) and molybdenum (M)
. Increased strength during heat treatment, corrosion resistance. - Aluminum (U)
. Increased resistance to scale when exposed to high temperatures. - Vanadium (F)
. Improving the structure, providing higher heat resistance.
Non-metallic additives are also added to alloy steels
:
- Manganese (G)
. Reducing the harmful effects of sulfur, phosphorus and oxygen. - Silicon (C)
. Increased strength while maintaining viscosity. - Selenium (E)
. Increased fluidity, easier mechanical processing. - Boron (R)
. Improved microstructure, increased hardenability. - Nitrogen (A)
. Providing improved mechanical properties - this component is added to high-alloy steels.
Undesirable impurities
The sulfur contained in the melt, mainly in the form of FeS, does quite a lot of harm to it. This element imparts red brittleness to steel - high-temperature brittleness, which manifests itself, for example, during forging. It also increases the metal's tendency to abrasion, reduces its resistance to fatigue, and negatively affects corrosion resistance. The negative impact of sulfur can be reduced by adding manganese to the alloying composition, which binds this element. Also, to neutralize sulfur, lanthanum, cesium, and neodymium are introduced into the metal.
Another significant harmful impurity is phosphorus. It reduces the toughness of steel at low temperatures - this property is called cold brittleness. However, the presence of phosphorus in the alloy promotes better chip separation, and this improves the machinability of the material.
Nitrogen and oxygen are usually present in the metal in a chemically bound form and enter the steel as part of the ore, fuel, or deoxidizer added to the charge. Their presence reduces the viscosity and ductility of the metal, they contribute to the formation of weak formations in the alloy - oxides, nitrides, which become stress concentrators in the metal mass and seriously impair the endurance and viscosity of the material, making it susceptible to brittle fracture. Nitrogen, in addition, can lead to strain aging of steel.
Hydrogen is a very undesirable impurity. The result of its presence in the metal in quantities exceeding the permissible:
- fragility of steel;
- formation of flakes - defects in the form of round or oval silver-white cracks;
- decrease in viscosity;
- deterioration or complete loss of plasticity.
A properly composed alloying composition and quality control of the alloy steel produced at all stages of its production make it possible to reduce the negative impact of harmful impurities to a minimum.
Types of Alloy Steel
There are three main categories of such steels, the classification of which takes into account the proportion of impurities and alloying additives.
- Low alloy steel
– its composition contains approximately 2.5% alloying elements. - Medium alloy steel
- includes 2.5–10% alloying substances. - High-alloy steel
- contains more than 10% of the additives we are interested in, and their content can reach up to 50%.
The properties of the metal depend on the proportion of carbon. If its amount is 0.25–2.14%, the steel is carbon and is classified as follows:
- high carbon: 0.6–2%;
- medium carbon: 0.3–0.6%;
- low carbon: no more than 0.25%.
Adding new components is impossible without removing some of the old ones, otherwise linking is impossible. Purification allows you to reduce the share of harmful impurities and oxygen. Carbon is removed by burning due to the precipitation of carbides and other methods. Additives can be added to any steel, but this procedure does not always give the desired result.
In alloy steel, the carbon component is indicated in hundredths of a percent. There is a classification of alloy steels based on the total mass of additives:
- low alloyed – up to 2.5%;
- medium alloyed – 2.5–10%;
- highly alloyed - from 10%.
Due to the content of additives in alloy steel, recrystallization and the formation of a new structure occur. Based on the shape of the crystal lattice, the following classes of steel are distinguished:
- Ferrites
. Magnetic, the lattice is unstable, changes as a result of heating, cooling, transforming into perlite, sorbitol, trostite. This group includes all low-alloy and carbon steels.
It is possible to ensure the formation of stable bonds by reducing the proportion of carbon to 0.15% and adding chromium as an alloying component.
- Austenites
. They are characterized by a high content of nickel, chromium and manganese. Due to their structural structure, they are heat-resistant, plastic, and are not afraid of rust. This group includes chromium-nickel stainless steels.
- Martensites
. Cooling after quenching leads to martensitic transformation, resulting in the formation of cubic cells that make up needle or lath crystals. The metal acquires memory, so it is able to partially recover after deformation.
Steels containing chromium, molybdenum, vanadium, tungsten, niobium and other components that provide heat resistance can go into this state.
The metal crystal lattice is organized in the form of phases - most often there are two phases at once. Let's say there can be austenite and ferrite. The required phase is increased using additives and exposure to temperature.
During smelting, cast iron is obtained from ore, which is refined, that is, purified from gases, oxides, and other inclusions. Oxygen is removed with coal, slag, manganese and other deoxidizers - they cause the formation of gases or heavy oxides that precipitate.
The process of decarburization, or carbon removal, from alloy steel uses hydrogen and burns out the carbides, which releases carbon monoxide and forms scale. At the moment, some enterprises use modern technologies, such as gas-oxygen refining.
The quality of the metal depends on the result of these procedures. Based on this feature, the following steels are distinguished:
- Ordinary, or ordinary
. This is the cheapest material with a carbon content of around 0.6%, while there are air bubbles in the metal. The most common brands are: STO, St3sp, St5kp. - High quality
. This includes calm, semi-calm and boiling species, which contain oxygen, nitrogen, and hydrogen. In this case, the maximum concentration of gases is achieved in boiling water. Steels can be carbon and alloyed grades St08kp, St10ps, St20, 7ХФ, 8ХФ. - High quality
. They are distinguished by a reduced content of sulfur and phosphorus - within 0.03%. These steels are smelted in electric furnaces without the use of coal. These include 6ХВ2С, 6Х3ФС. - Particularly high quality
. Hot metal undergoes deep cleaning to remove oxides, sulfides, and non-metallic inclusions. As a result, up to 0.01% sulfur and 0.025% phosphorus remain in it. We are talking, for example, about a brand such as 30ХГС3-Ш.
In addition, there is a classification of alloy steels based on their purpose
:
Structural
They are used for the production of building structures and loaded mechanisms.
Types of structural alloy steels:
- Improveable. They stand out against the general background due to their high chromium content, enriched with boron, nickel, molybdenum, manganese, and are used for heat treatment.
- Spring-spring. They contain added silicon, cobalt, manganese, boron, titanium, and are used in the production of transport.
- Bearing. They are characterized by increased hardness and wear resistance, always contain chromium and a minimum content of non-metallic additives.
- Heat resistant. Used in the production of steam heaters.
Tool (cutting and stamping)
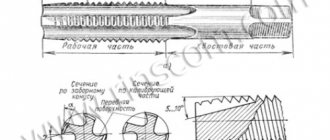
Additives added to tool alloy steels are responsible for increased strength and uniformity. Most often, the metal undergoes heat treatment and is used for the manufacture of cutters, cutters, and taps. Alloying is carried out with chromium, vanadium, titanium and other components.
Such steels are very expensive and high-speed, which is why they are used exclusively in cutting planes. For measuring instruments, chromium, tungsten, and manganese are added to the metal, ensuring its hardness and dimensional stability.
Steels with special properties, namely stainless, heat-resistant, wear-resistant, etc.
We are talking about a significant group, the metals in which have different properties:
- High-strength – high-alloy steels with a selected composition, thanks to which the metal is used for the production of critical components of mechanisms.
- Stainless steel - includes manganese, chromium, suitable for working in chemically aggressive environments, used for the production of pipes.
- Wear-resistant - characterized by a high proportion of manganese. They are used to make railroad switches, tracks, mining equipment, and excavator buckets.
In addition to the mentioned steels, this group includes heat-resistant, heat-resistant, magnetic, non-magnetic, rheostatic, and with high electrical resistance.
Modern alloys are complex alloyed compounds with unique characteristics. Thus, 15Kh2NMFA steel is designed to ensure the radiation life of a reactor installation for 100 years, and 17KhNGT is used as a material for special-purpose springs.
Metal alloying process
In most cases, steel alloying elements are added to commercially pure metal. In the molten state, the addition of additives occurs particularly quickly, so most grades of alloy steels and alloys are produced by melting a base metal, such as iron, aluminum or copper, and then adding alloying components.
Care must be taken to avoid contamination by foreign matter. In fact, refining and alloying are performed simultaneously. This is how the steelmaking industry desulfurizes liquid blast iron in a ladle, decarburizes the iron during its conversion to steel, removes oxygen from the liquid steel in a vacuum degasser, and finally adds the required amount of alloying additives to the original melt.
The largest volumes of alloys are melted in air, and slag is used to protect the metal from oxidation. With a large volume of alloying elements, the process is carried out directly in a ladle or in a vacuum chamber. This allows for careful control of the composition and minimizes metal oxidation.
Most of the alloying elements are located in the initial charge, and melting occurs using electric and induction heating, or by melting with an electric arc. Induction melting is carried out in a crucible, and in electric arc melting, molten drops of metal flow from the electrode onto a water-cooled surface and immediately solidify.
Marking of alloy steels
The grades are designated using an alphanumeric marking system for alloy steels. In other words, each brand is identified by a combination of letters and numbers.
Thus, elements added to alloy steel are designated by letters of the Russian alphabet, where X is chromium, N is nickel, B is tungsten, M is molybdenum, F is vanadium, T is titanium, Y is aluminum, D is copper, G is manganese , C – silicon, K – cobalt, C – zirconium, P – boron, B – niobium.
The letter A in the middle of the mark indicates the nitrogen content, and at the end indicates that the steel is high quality.
For structural steels, the first pair of digits of the marking can be used to understand the carbon content, which is indicated in hundredths of a percent.
When the amount of alloying element exceeds 1%, the average value in whole percentage is written after the letter. If about 1% or less of this component is added, the figure is not given.
Thus, 18KhGT steel contains (in percent): 0.18 C, 1 Cr, 1 Mn, about 0.1 Ti. The marking of alloy steel 38KhNZMFA can be deciphered as: 0.38 C, 1.2–1.5 Cr; 3 Ni, 0.3–0.4 Mo, 0.1–0.2 V. 30ХГСA includes: 0.30 C, 0.8–1.1 Cr, 0.9–1.2 Mn, 0. 8–1.251. And OZKh13AG19 steel includes 0.03 C, 13 Cr, 0.2–0.3 N. 19 Mn.
Recommended articles
- High-speed steel - characteristics, markings, applications
- Corrosion-resistant steel: types and features
- Metal hardening: technology and choice of temperature conditions
For tool steels, the grade begins with a number that indicates the amount of carbon in tenths of a percent. This figure is not written when the content of this component is 1% or higher.
Let’s say that 3Kh2V8F steel contains 0.3 C, 2 Cr, 8 XV, 0.2–0.5 V. Whereas the 5KhNM marking stands for: 0.5 C, 1 Cr, 1 N1, up to 0.3 Mo. And in CHG there are 1 C, 1 Cr, 1 TC, 1 Mn.
For some steel groups there are additional designations. So, in grades of automatic steels the letter A comes first, in bearing grades it is the letter Ш, in high-speed steels it is P, in electrical steels it is E, in magnetic-hard steels it is E.
Applications of alloy steel
Alloy steel is widely used in industry today. Its high strength allows it to be used in the production of equipment for cutting and chopping different types of rolled metal.
At the moment, alloy steels are used in a variety of fields, here are some of them:
- medical instruments, including sharp cutting objects;
- blades;
- bearings, parts experiencing high radial and support loads;
- cutters, cutters, drills, other equipment for machine tools in the field of metalworking;
- housings for equipment and devices;
- stainless steel utensils, such as buckets, basins, etc.;
- parts for the automotive industry.
From a practical point of view, among alloy steels there are:
- Mechanical engineering, used for the production of mechanism parts and body structures. They must be subjected to heat treatment.
- Construction materials, they are most often used for the manufacture of welded metal structures and only in rare cases are they subjected to strong heating.
This alloy steel is a material for three groups of tools:
- cutting;
- measuring;
- stamps.
Low-alloy steel is used to produce the bodies of railway cars, subway cars, trams, load-bearing structures of locomotives, agricultural and other field machines. This steel also serves as a material for engineering structures operating under variable dynamic loads, seasonal and daily heat changes.
Alloy steels can have different properties, which they obtain due to the ratio of the main elements. However, you need to understand how any alloy steel differs. What makes it stand out from the general background is its increased strength and resistance to rust formation.
Welding alloys
Alloy alloys are flexible and are used to make complex mechanisms by welding. Due to the different content of impurities, each type of alloy alloys has its own specific characteristics.
Interesting: Types and equipment of metal slitting
Low alloy
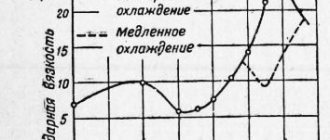
These metals are often hardened and weld well, but their seams do not withstand excessive stress. It is necessary to preheat the alloy and cool it slowly to prevent cold cracks from forming.
Medium alloyed
Alloy steels of this type are obtained reliable when components made of tungsten, molybdenum, and vanadium are used. It is necessary to select electrodes with the same substances, but not at the same concentration. The alloy requires protection from overheating, oxidation processes, and hydrogen disease.