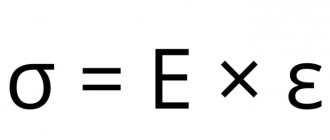Plasticity of metals.
Section: LIBRARY OF TECHNICAL LITERATURE Short path https://bibt.ru<<Previous page Book table of contents Next page>>
Plasticity is the ability of a metal to take on a new shape under load without breaking.
The ductility of metals is also determined by tensile testing. This property is revealed in the fact that under the influence of a load, samples of different metals elongate to varying degrees, and their cross-section decreases. The more the sample is able to elongate and its cross section to narrow, the more ductile the sample metal is.
The need to determine the ductility of metals is caused by the fact that ductile metals can be subjected to pressure treatment, i.e., forged, stamped, or metal ingots can be converted into strips, sheets, rods, rails, and many other products and workpieces on rolling mills.
In contrast to ductile metals, brittle metals are destroyed under load without changing shape. During testing, brittle samples are destroyed without elongation, suddenly. Fragility is a negative property. Not only durable, but also to a certain extent ductile metal will be quite suitable for the manufacture of machine parts.
In order to get an idea of the ductility of a metal and determine the value of this property, there are two units of measurement: relative elongation and relative contraction at break.
The value of relative elongation is determined during testing as follows.
First, the total elongation of the sample at rupture l1-l0 is calculated, i.e., from its length at the moment of rupture l1, the initial length l0 is calculated. The resulting difference could serve as an indicator of the ductility of metals only if the length of the test samples were always the same.
When the initial length of the samples is different, the magnitude of their elongation for comparing the ductility of metals is insufficient, since long samples will elongate at break more than short samples from the same metal.
Therefore, in order to be able to compare the ductility of different metals, it is necessary to take into account what the initial length of the sample is and what elongation it received at break relative to its original length.
Relative elongation is usually expressed numerically as a percentage relative to the original length of the sample and is denoted by the letter δn.
Example: Initial sample length l0 = 200 mm; the length at break was 236 mm; the elongation of the sample was 236–200 = 36 mm. Relative extension
The relative elongation (%) when testing some metals is: for zinc 20, aluminum 40, tin 40, iron 45, lead 45, nickel 50, copper 50.
The second quantity characterizing the plasticity of metals, the relative contraction at break ψ, is determined in a similar way:
where F0 is the initial cross-sectional area of the sample before testing, mm2; F1 is the cross-sectional area of the sample at the fracture site, mm2.
Thus, the relative contraction is the ratio of the amount of reduction in the cross-sectional area of the sample upon rupture to the original cross-sectional area.
Skip to navigation
Characteristics of technological properties of metals and alloys
The deformation of metals and alloys is determined by their properties, which are considered technological, since they determine the technological regime of their pressure treatment. These include ductility, resistance to deformation, sensitivity to stress, and propensity to form defects.
The plasticity of a metal is its ability to deform under the influence of applied external forces without breaking its continuity. When processing metals by pressure (rolling, forging, pressing, etc.), the plasticity of the metal is affected by the degree of deformation (compression), deformation temperature, deformation rate, chemical composition of the metal and its structure, the nature of the stress state during deformation, etc.
An indicator of plasticity is the degree of deformation, expressed as relative compression. To assess plasticity, Yu. M. Chizhikov introduced the concept of “plasticity limit,” which is characterized by the magnitude of the relative compression at which the discontinuity of the metal begins. The higher the plasticity limit of a metal over a wide temperature range, the greater the margin of plasticity it has and the easier it is to deform. Therefore, the higher the plasticity of the metal, the greater the degree of total compression e it can withstand without destruction.
Depending on the value of the plasticity limit, metals and alloys are conventionally distinguished: the highest plasticity (>0.8 ε); high ductility (0.6÷0.8 ε); medium ductility (0.4÷0.6 ε); reduced ductility (0.2÷0.4 ε); low ductility (≤0.2 ε). According to their plastic properties, many grades of steel are classified as high and even the highest ductility. Most alloy steels fall into the categories of medium and high ductility. High-alloy steels and alloys have average and, in some cases, reduced ductility.
There is an opinion that if a metal is “soft”, then it can be deformed without destruction, i.e. it is ductile. This is not always correct. A soft metal, having a low resistance to deformation, under certain conditions may not be ductile, and, conversely, a “hard” metal, i.e., a metal with a high resistance to deformation, may have high plastic properties. For example, commercially pure iron, which has a very low resistance to deformation, is not ductile at a temperature of 1000–1050 °C and is destroyed when deformed, and high-speed steel, having a resistance to deformation 2–3 times greater than commercially pure iron, is deformed at the same temperatures without destruction.
Resistance to deformation is a very important technological property. Under the same deformation conditions, the higher the deformation resistance of a particular metal or alloy, the more difficult it is to roll. Steels with greater resistance to deformation must be rolled with smaller reductions or more powerful equipment must be used for this, etc.
Resistance to deformation largely depends on the chemical composition of the steel. The influence of the chemical composition of steel on the resistance to deformation occurs through a change in its mechanical properties (tensile strength and yield), as well as through a change in the coefficient of friction.
The plasticity of metals and alloys is determined by tension (elongation and compression), twisting, impact bending, upsetting, and wedge rolling. The plasticity indicators are as follows: during tension - relative elongation δ, % and relative compression ψ, %; for impact bending - impact strength an, J/m2; when twisting - the number of twists before breaking; during upsetting and click rolling - relative compression ∇h/h0.
In tensile testing, specimens are placed in the clamps of a tensile testing machine and stretched under increasing loads until they break completely. During this test, the tensile strength σв, Pa, and the yield strength σт, Pa, which are of great importance in determining the resistance of a metal to deformation, are determined. Tests are carried out at various temperatures (for example, from 20° to 1300°C). The tensile test meets the conditions corresponding to the linear stress state.
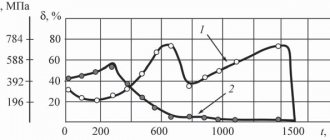
In Fig. Figure 48 shows the curves of relative elongation δ and compression ψ of the alloy tested at temperatures from 20° to 1100°C. The curves show that at 700°C both plasticity indices have the lowest value. With a further increase in temperature, elongation and compression increase, reaching the same values at 1000 °C as at room temperature, and significantly exceeding them at higher temperatures.
Plasticity indices obtained by the tensile method provide important characteristics of the ductility of alloys. By comparing the elongation and compression curves of different alloys or the same alloy melted by different methods, it is possible to establish which alloy or melt has greater or less ductility and what are the general plastic properties of a given steel or alloy. It should be noted that plasticity indicators obtained by the tensile method do not make it possible to sufficiently accurately determine the temperature of hot deformation (rolling, forging, pressing).
When testing for impact bending, samples of square cross-section with a notch in the middle of the length are heated to the test temperature. The samples are fractured using a copter.
In Fig. Figure 49 shows the impact strength an curve of one alloy, from which it can be established that the best deformation temperature for the alloy is 1150–1200°C.
The torsion test is carried out on an apparatus equipped with an electric tube furnace. One end of the sample is fixed motionless, the other rotates from an appropriate drive. The sample at a given temperature is subjected to twisting until it breaks. The higher the ductility, the greater the number of 360° twists the metal can withstand before breaking. In Fig. Figure 50 shows the curves obtained by hot torsion testing of steel grades 15, 40ХН, У10. Using these curves, you can determine the heating temperature of the metal at which the highest ductility is achieved.
When testing by the wedge rolling method, samples are used, manufactured to the required dimensions by casting, rolling or forging from ingots. The relative compression at which discontinuity begins is the plasticity limit of the metal under given conditions. By studying the plasticity of a metal using the wedge rolling method in a wide temperature range, it is possible to obtain a complete characteristic of the plasticity of a metal with any natural properties when rolling it with free broadening, i.e. under the most unfavorable deformation conditions.
Based on the results of studying plasticity using the wedge rolling method, it is possible to establish the temperature regime for the beginning and end of rolling and the permissible reductions.
Metal properties
Why do jewelry makers and repairers put gold first? First of all, this is due to its excellent ductility: from 1 gram of metal, a wire up to 3 kilometers long can be drawn, gold ingots are forged into sheets, the thickness of which is measured in ten thousandths of a millimeter. The domes of temples are covered with this gold; it is called leaf. It looks quite interesting: when exposed to light it gives a blue-green tint.
Pure gold can dissolve in aqua regia. This is the name given to a mixture of two concentrated acids: nitric and hydrochloric. The most ductile metal in the table is number 79, melting point is 1064 ° C, density is 19.32 g/cm3. In terms of thermal conductivity and electrical resistance, gold is second only to silver and copper.
Gold in its pure form is too soft, so jewelry is usually made from alloys. Most often, silver or copper is added to gold. Have you ever wondered what “test” means on jewelry? This is the pure gold content in parts per thousand. 999 purity is considered pure gold.
Application
Gold has long been used as an investment object; in addition, it has found active use in the jewelry industry.
In many countries, gold coins were used as money. Despite this, it was recognized as a world currency only in the 19th century. In 1922, bank notes with gold content, called “chervonets”, appeared in circulation in Russia. One bank note was equivalent to 10 gold rubles of the old coinage.
Gold is the most common material used in jewelry making. The higher the gold purity, the better resistance to corrosion the material will have; silver and copper give the product strength and different shades of color.