The leading trends in modern construction are the construction of houses with maximum energy efficiency. That is, with the ability to create and maintain comfortable living conditions with minimal energy costs. It is clear that many of our builders, who are constructing their residential properties on their own, are still far from achieving such indicators, but it is always necessary to strive for this.
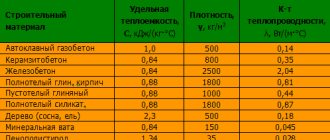
Thermal conductivity of building materials
First of all, this concerns minimizing heat losses through building structures. This reduction is achieved by effective thermal insulation, performed on the basis of thermal engineering calculations. Design should ideally be carried out by specialists, but often circumstances force homeowners to take such matters into their own hands. This means that it is necessary to have a general understanding of the basic concepts of building heating engineering. First of all, what is the thermal conductivity of building materials, how is it measured, and how is it calculated.
If you understand these “basics”, then it will be easier to seriously, with knowledge of the matter, and not on a whim, to deal with the issues of insulating your home.
General concept of thermal conductivity and its nature
If we answer in simple words the question of what thermal conductivity is in physics, then it should be said that the transfer of heat between two bodies or different regions of the same body is the process of exchange of internal energy between the particles that make up the body (molecules, atoms, electrons and ions). Internal energy itself consists of two important parts: kinetic and potential energy.
What is thermal conductivity in physics in terms of the nature of this quantity? At the microscopic level, the ability of materials to conduct heat depends on their microstructure. For example, for liquids and gases, this physical process occurs due to chaotic collisions between molecules; in solids, the main share of the transferred heat is due to the exchange of energy between free electrons (in metallic systems) or phonons (non-metallic substances), which are mechanical vibrations of the crystal lattice .
Notes
- [dic.academic.ru/dic.nsf/natural_science/14229/FOURIER Natural history. Encyclopedic Dictionary. Fourier's law.]
- D.V.
Sivukhin. General physics course: thermodynamics and molecular physics. - M.: Fizmatlit, 2006. - P. 345. - [ustu.ru/fileadmin/user_upload/kafedra_fiziki/pdf/3b.pdf Study of thermal conductivity of gases.] // Methodological instructions.
- J. C. Maxwell, Philos. Trans. Roy. Soc. London 157
(1867) 49. - C. Cattaneo, Atti Seminario Univ. Modena 3
(1948) 33.
Methods of transferring thermal energy
When considering the question of what the thermal conductivity of materials is, it is worth mentioning possible methods of heat transfer. Thermal energy can be transferred between different bodies through the following processes:
- conductivity - this process occurs without the transfer of matter;
- convection - heat transfer is directly related to the movement of matter itself;
- radiation - heat transfer is carried out due to electromagnetic radiation, that is, with the help of photons.
For heat to be transferred through the processes of conduction or convection, direct contact between different bodies is necessary, with the difference that in the process of conduction there is no macroscopic movement of matter, but in the process of convection this movement is present. Note that microscopic motion occurs in all heat transfer processes.
For ordinary temperatures of a few tens of degrees Celsius, it can be said that convection and conduction account for the bulk of the heat transferred, and the amount of energy transferred by radiation is negligible. However, radiation begins to play a major role in the process of heat transfer at temperatures of several hundred and thousand Kelvin, since the amount of energy Q transferred in this way increases in proportion to the 4th power of absolute temperature, that is, ∼ T4. For example, our sun loses most of its energy through radiation.
Generalizations of Fourier's law
It should be noted that Fourier’s law does not take into account the inertia of the thermal conduction process, that is, in this model, a change in temperature at some point instantly spreads to the entire body. Fourier's law is not applicable to describe high-frequency processes (and, accordingly, processes whose Fourier series expansion has significant high-frequency harmonics). Examples of such processes are the propagation of ultrasound, shock waves, etc. Maxwell was the first to introduce inertia into the transport equations [4], and in 1948 Cattaneo proposed a version of Fourier’s law with a relaxation term: [5]
\tau\frac{\partial\mathbf{q}}{\partial t}=-\left(\mathbf{q}+\varkappa\,\nabla T\right).
If the relaxation time \tau is negligible, then this equation becomes Fourier's law.
Thermal conductivity coefficient for solids
The coefficient of thermal conductivity for solids k has the following physical meaning: it indicates the amount of heat that passes per unit time through a unit surface area in a body of unit thickness and infinite length and width with a temperature difference at its ends equal to one degree. In the international system of SI units, the coefficient k is measured in J/(s*m*K).
This coefficient in solids depends on temperature, so it is usually determined at a temperature of 300 K in order to compare the ability to conduct heat of different materials.
Thermal conductivity coefficient for metals and non-metallic solids
All metals, without exception, are good conductors of heat, the transfer of which into them is carried out by electron gas. In turn, ionic and covalent materials, as well as materials with a fibrous structure, are good thermal insulators, that is, they conduct heat poorly. To fully explain the question of what thermal conductivity is, it should be noted that this process requires the presence of a substance if it is carried out through convection or conduction, therefore, in a vacuum, heat can only be transferred due to electromagnetic radiation.
The list below shows the values of thermal conductivity coefficients for some metals and non-metals in J/(s*m*K):
- steel - 47-58 depending on the steel grade;
- aluminum - 209.3;
- bronze - 116-186;
- zinc - 106-140 depending on purity;
- copper - 372.1-385.2;
- brass - 81-116;
- gold - 308.2;
- silver - 406.1-418.7;
- rubber - 0.04-0.30;
- fiberglass - 0.03-0.07;
- brick - 0.80;
- wood - 0.13;
- glass - 0.6-1.0.
Thus, the thermal conductivity of metals is 2-3 orders of magnitude higher than the thermal conductivity values for insulators, which are a clear example of the answer to the question of what low thermal conductivity is.
Thermal conductivity plays an important role in many industrial processes. In some processes, they try to increase it by using good thermal conductors and increasing the contact area, while in others they try to reduce thermal conductivity by reducing the contact area and using heat-insulating materials.
Convection in liquids and gases
Heat transfer in fluids occurs through the process of convection. This process involves the movement of molecules of a substance between zones with different temperatures, that is, during convection, mixing of a liquid or gas occurs. When fluid matter gives off heat, its molecules lose some of their kinetic energy, and the matter becomes denser. On the contrary, when fluid matter heats up, its molecules increase their kinetic energy, their movement becomes more intense, accordingly, the volume of matter increases and density decreases. That is why cold layers of matter tend to fall down under the influence of gravity, and hot layers try to rise up. This process causes the matter to mix, facilitating the transfer of heat between its layers.
We recommend: 10 best steel heating radiators - 2020 rating and tips for choosing
Factors affecting thermal conductivity
The thermal conductivity coefficient of a material depends on several factors:
- As this indicator increases, the interaction between material particles becomes stronger. Accordingly, they will transmit temperature faster. This means that as the density of the material increases, heat transfer improves.
- Porosity of a substance. Porous materials are heterogeneous in their structure. There is a large amount of air inside them. This means that it will be difficult for molecules and other particles to move thermal energy. Accordingly, the thermal conductivity coefficient increases.
- Humidity also affects thermal conductivity. Wet surfaces of the material transmit more heat. Some tables even indicate the calculated thermal conductivity coefficient of the material in three states: dry, medium (normal) and wet.
When choosing a material for insulating rooms, it is also important to take into account the conditions in which it will be used.
Material temperature
The effect of temperature on the ability to conduct heat differs for metals and nonmetals.
In metals, conductivity is mainly due to free electrons. According to the Wiedemann-Franz law, the thermal conductivity of a metal is proportional to the product of the absolute temperature, expressed in Kelvin, and its electrical conductivity. In pure metals, electrical conductivity decreases with increasing temperature, so thermal conductivity remains approximately constant. In the case of alloys, electrical conductivity changes little with increasing temperature, so the thermal conductivity of alloys increases in proportion to temperature. On the other hand, heat transfer in nonmetals is mainly associated with lattice vibrations and the exchange of lattice phonons. With the exception of high-quality crystals and low temperatures, the path of phonons in the lattice does not decrease significantly at high temperatures, and therefore the thermal conductivity remains constant over the entire temperature range, that is, it is insignificant. At temperatures below the Debye temperature, the ability of nonmetals to conduct heat, along with their heat capacity, decreases significantly.
Phase transitions and structure
When a material undergoes a first-order phase transition, for example from a solid to a liquid or from a liquid to a gas, its thermal conductivity may change. A striking example of such a change is the difference between this physical quantity for ice (2.18 W/(m*K) and water (0.90 W/(m*K).
Changes in the crystal structure of materials also affect thermal conductivity, which is explained by the anisotropic properties of various allotropic modifications of a substance of the same composition. Anisotropy affects different scattering intensities of lattice phonons, the main heat carriers in nonmetals, and in different directions in the crystal. A striking example here is sapphire, whose conductivity varies from 32 to 35 W/(m*K) depending on the direction.
Electrical conductivity
Thermal conductivity in metals changes along with electrical conductivity according to the Wiedemann-Franz law. This is due to the fact that valence electrons, moving freely throughout the crystal lattice of the metal, transfer not only electrical, but also thermal energy. For other materials, the correlation between these types of conductivity is not pronounced, due to the insignificant contribution of the electronic component to thermal conductivity (in nonmetals, lattice phonons play the main role in the mechanism of heat transfer).
Convection process
Air and other gases are, as a rule, good heat insulators in the absence of convection. This principle is the basis for the operation of many heat-insulating materials containing a large number of small voids and pores. This structure does not allow convection to spread over long distances. Examples of such man-made materials are polystyrene and silicide airgel. In nature, heat insulators such as animal skin and bird plumage work on the same principle.
Light gases such as hydrogen and gel have high thermal conductivities, while heavy gases such as argon, xenon and radon are poor conductors of heat. For example, argon, an inert gas that is heavier than air, is often used as an insulating gas filler in double-glazed windows and light bulbs. An exception is sulfur hexafluoride (SF6 gas), which is a heavy gas and has a relatively high thermal conductivity due to its high heat capacity.
Table of thermal conductivity of thermal insulation materials
To make it easier to keep your house warm in winter and cool in summer, the thermal conductivity of walls, floors and roofs must be at least a certain figure, which is calculated for each region. The composition of the “pie” of walls, floor and ceiling, the thickness of the materials are taken into account so that the total figure is no less (or better yet, at least a little more) recommended for your region.
Heat transfer coefficient of modern building materials for enclosing structures
When choosing materials, it is necessary to take into account that some of them (not all) conduct heat much better in conditions of high humidity. If such a situation may occur for a long period of time during operation, the thermal conductivity for this condition is used in the calculations. The thermal conductivity coefficients of the main materials used for insulation are given in the table.
Name of material /Thermal conductivity coefficient W/(m °C)
| Dry | At normal humidity | At high humidity | |
| Woolen felt | 0,036-0,041 | 0,038-0,044 | 0,044-0,050 |
| Stone mineral wool 25-50 kg/m3 | 0,036 | 0,042 | 0,,045 |
| Stone mineral wool 40-60 kg/m3 | 0,035 | 0,041 | 0,044 |
| Stone mineral wool 80-125 kg/m3 | 0,036 | 0,042 | 0,045 |
| Stone mineral wool 140-175 kg/m3 | 0,037 | 0,043 | 0,0456 |
| Stone mineral wool 180 kg/m3 | 0,038 | 0,045 | 0,048 |
| Glass wool 15 kg/m3 | 0,046 | 0,049 | 0,055 |
| Glass wool 17 kg/m3 | 0,044 | 0,047 | 0,053 |
| Glass wool 20 kg/m3 | 0,04 | 0,043 | 0,048 |
| Glass wool 30 kg/m3 | 0,04 | 0,042 | 0,046 |
| Glass wool 35 kg/m3 | 0,039 | 0,041 | 0,046 |
| Glass wool 45 kg/m3 | 0,039 | 0,041 | 0,045 |
| Glass wool 60 kg/m3 | 0,038 | 0,040 | 0,045 |
| Glass wool 75 kg/m3 | 0,04 | 0,042 | 0,047 |
| Glass wool 85 kg/m3 | 0,044 | 0,046 | 0,050 |
| Expanded polystyrene (foam plastic, EPS) | 0,036-0,041 | 0,038-0,044 | 0,044-0,050 |
| Extruded polystyrene foam (EPS, XPS) | 0,029 | 0,030 | 0,031 |
| Foam concrete, aerated concrete with cement mortar, 600 kg/m3 | 0,14 | 0,22 | 0,26 |
| Foam concrete, aerated concrete with cement mortar, 400 kg/m3 | 0,11 | 0,14 | 0,15 |
| Foam concrete, aerated concrete with lime mortar, 600 kg/m3 | 0,15 | 0,28 | 0,34 |
| Foam concrete, aerated concrete with lime mortar, 400 kg/m3 | 0,13 | 0,22 | 0,28 |
| Foam glass, crumbs, 100 - 150 kg/m3 | 0,043-0,06 | ||
| Foam glass, crumbs, 151 - 200 kg/m3 | 0,06-0,063 | ||
| Foam glass, crumbs, 201 - 250 kg/m3 | 0,066-0,073 | ||
| Foam glass, crumbs, 251 - 400 kg/m3 | 0,085-0,1 | ||
| Foam block 100 - 120 kg/m3 | 0,043-0,045 | ||
| Foam block 121-170 kg/m3 | 0,05-0,062 | ||
| Foam block 171 - 220 kg/m3 | 0,057-0,063 | ||
| Foam block 221 - 270 kg/m3 | 0,073 | ||
| Ecowool | 0,037-0,042 | ||
| Polyurethane foam (PPU) 40 kg/m3 | 0,029 | 0,031 | 0,05 |
| Polyurethane foam (PPU) 60 kg/m3 | 0,035 | 0,036 | 0,041 |
| Polyurethane foam (PPU) 80 kg/m3 | 0,041 | 0,042 | 0,04 |
| Cross-linked polyethylene foam | 0,031-0,038 | ||
| Vacuum | |||
| Air +27°C. 1 atm | 0,026 | ||
| Xenon | 0,0057 | ||
| Argon | 0,0177 | ||
| Airgel (Aspen aerogels) | 0,014-0,021 | ||
| Slag | 0,05 | ||
| Vermiculite | 0,064-0,074 | ||
| Foam rubber | 0,033 | ||
| Cork sheets 220 kg/m3 | 0,035 | ||
| Cork sheets 260 kg/m3 | 0,05 | ||
| Basalt mats, canvases | 0,03-0,04 | ||
| Tow | 0,05 | ||
| Perlite, 200 kg/m3 | 0,05 | ||
| Expanded perlite, 100 kg/m3 | 0,06 | ||
| Linen insulating boards, 250 kg/m3 | 0,054 | ||
| Polystyrene concrete, 150-500 kg/m3 | 0,052-0,145 | ||
| Granulated cork, 45 kg/m3 | 0,038 | ||
| Mineral cork on a bitumen basis, 270-350 kg/m3 | 0,076-0,096 | ||
| Cork flooring, 540 kg/m3 | 0,078 | ||
| Technical cork, 50 kg/m3 | 0,037 |
We recommend: Electric heaters for heating with minimal energy consumption
Some of the information is taken from standards that prescribe the characteristics of certain materials (SNiP, SP, SNiP II-3-79* (Appendix 2)). Those materials that are not specified in the standards are found on the manufacturers' websites. Since there are no standards, they can differ significantly from different manufacturers, so when purchasing, pay attention to the characteristics of each material you purchase.
Application of thermal conductivity in practice
In construction, all materials are conventionally divided into thermal insulation and structural. Structural raw materials have the highest thermal conductivity, but it is precisely this material that is used for the construction of walls, ceilings, and other fences. According to the table of thermal conductivity of building materials, when constructing walls made of reinforced concrete, for low heat exchange with the environment, the thickness of the structure should be about 6 meters. In this case, the structure will turn out to be huge, cumbersome and will require considerable costs.
A clear example is at what thickness of different materials their thermal conductivity coefficient will be the same
Therefore, when constructing a building, special attention should be paid to additional heat-insulating materials. A layer of thermal insulation may not be needed only for buildings made of wood or foam concrete, but even when using such low-conductivity raw materials, the thickness of the structure must be at least 50 cm.
Need to know! Thermal insulation materials have minimal thermal conductivity values.
Features of thermal conductivity of the finished structure
When planning the design of your future home, you must take into account possible losses of thermal energy. Most of the heat escapes through doors, windows, walls, roofs and floors.
In apartment buildings, heat loss will differ compared to a private building
If you do not carry out calculations for heat conservation at home, the room will be cool. It is recommended that buildings made of brick, concrete and stone be additionally insulated.
Insulation of buildings made of concrete or stone increases comfortable conditions inside the building
Helpful advice! Before insulating your home, you need to consider high-quality waterproofing. Moreover, even high humidity will not affect the thermal insulation properties of the room.
Types of insulation of structures
A warm building will be achieved with the optimal combination of a structure made of durable materials and a high-quality heat-insulating layer. Such structures include the following:
- When constructing a frame building, the wood used ensures the rigidity of the building. The insulation is laid between the racks. In some cases, insulation is applied to the outside of the building;
Installation work on insulating a frame structure requires the use of additional structural elements
- building made of standard materials: cinder blocks or bricks. In this case, insulation is often carried out on the outside.
Features of installing heat-insulating material from the inside
When is the thermal conductivity coefficient taken into account?
Thermal conductivity parameters must be taken into account when choosing materials for enclosing structures - walls, ceilings, etc. In rooms where the walls are made of materials with high thermal conductivity, it will be quite cool in the cold season. Decorating the room won't help either. In order to avoid this, the walls must be made quite thick. This will certainly lead to increased costs for materials and labor.
Insulation scheme for a wooden house
That is why the construction of the walls requires the use of materials with low thermal conductivity (mineral wool, polystyrene foam, etc.).
Characteristics of different materials
Before considering the table of thermal conductivity of insulation, it makes sense to read a brief overview. The information will help developers understand the specifics of the material and its purpose.
Styrofoam
Polystyrene foam and expanded polystyrene differ in their production method, price and thermal conductivity
. Board material made by foaming polystyrene. It is distinguished by ease of cutting and installation, low thermal conductivity - compared to other insulators, foam plastic is lighter. The advantages of the product are low cost, resistance to humid environments. Disadvantages of polystyrene foam: fragility, rapid flammability. For this reason, slabs with a thickness of 20-150 mm are used for thermal insulation of light external structures - facades for plastering, walls of plinths and basements.
When polystyrene foam burns, toxic substances are released.
Extruded polystyrene foam
Extruded polystyrene foam is resistant to moisture. The material is easy to cut, does not burn, and is easy to install and transport. In addition to low thermal conductivity, the slabs have high density and compressive strength. Extruded polystyrene foam of the Technoplex and Penoplex brands is popular among Russian developers. It is used for thermal insulation of blind areas and strip foundations.
Mineral wool
The denser the slabs of mineral basalt wool, the worse they conduct heat.
The thermal conductivity coefficient of mineral wool is 0.048 W/(m*C), which is higher than polystyrene foam. The material is made on the basis of rocks, slag or dolomite in the form of slabs and rolls, which have different stiffness indexes. To insulate vertical surfaces, it is allowed to use rigid and semi-rigid products. It is better to insulate horizontal structures using lightweight mini-plates.
Despite the optimal thermal conductivity index, mineral wool has little resistance to a humid environment. The slabs are not suitable for insulating basements, steam rooms, and dressing rooms.
The use of mineral wool with low thermal conductivity is allowed only if there is a vapor barrier and waterproofing layers.
Basalt wool
The basis for insulation is a type of basalt rock that swells when heated into fibers. Non-toxic binding components are also added during manufacturing. Rockwool brand products are on the Russian market, using the example of which you can consider the features of insulation:
- not subject to fire;
- has good thermal and sound insulation;
- absence of caking and compaction during operation;
- environmentally friendly building material.
Thermal conductivity parameters allow the use of stone wool for external and internal work.
Glass wool
Glass wool has a thermal conductivity coefficient higher than stone wool, the material is hygroscopic.
Glass wool insulation is made from borax, limestone, soda, sifted dolomite and sand. To save on production, glass cullet is used, which does not affect the properties of the material. The advantages of glass wool include high heat and sound insulation, environmental friendliness and low cost. More cons:
- Hygroscopicity - absorbs water, as a result of which it loses its insulating characteristics. To prevent rotting and destruction, structures are laid between vapor barrier layers.
- Inconvenient installation - fibers with increased fragility disintegrate and can cause burning and itching of the skin.
- Short-term use - shrinkage occurs after 10 years.
- Impossibility of use for insulation of wet rooms.
When working with glass wool, you need to protect your hands with gloves and your face with goggles or a mask.
Foamed polyethylene
Foamed foil polyethylene transmits heat worse than regular
polyethylene rolls with a porous structure and has an additional reflective layer of foil. Advantages of isolon and penofol:
- small thickness - from 2 to 10 mm, which is 10 times less than conventional insulators;
- the ability to retain up to 97% of useful heat;
- resistance to moisture;
- minimal thermal conductivity due to pores;
- environmental cleanliness;
- reflective effect due to which thermal energy is accumulated.
Rolled thermal insulation is suitable for installation in damp rooms, on balconies and loggias.
Spray insulation
Polyurethane foam has the lowest thermal conductivity.
If you look at the table, you can see that sprayed types replace 10 cm of mineral wool. They are produced in cylinders, resemble polyurethane foam and are applied using a special tool. Sprayed insulation comes in different hardnesses; the container also contains foaming agents - polyisocyanate and polyol. According to the type of main component, insulation is:
- PPU. Polyurethane foam with an open cell structure is durable and thermally efficient. If there are closed voids in the composition, steam can pass through.
- Foam insulation. Liquid polystyrene foam based on urea-formaldehyde is characterized by vapor permeability and fire resistance. Apply by pouring. The optimal hardening temperature is from +15 degrees.
- Liquid ceramics. Ceramic components are melted to a liquid state, then mixed with polymer substances and pigments. The result is evacuated cavities. External insulation provides protection for the building for 10 years, internal insulation for 25 years.
- Ecowool. Cellulose is crushed to dust and becomes sticky when exposed to water. The material is suitable for use on damp wall surfaces, but is not used near fireplace pipes, chimneys and stoves.
Sprayed insulation is characterized by good adhesion to surfaces for which wood, brick or aerated concrete were used.
Thermal conductivity coefficient of building materials: how it is used in practice and table
The practical value of the coefficient is a correctly carried out calculation of the thickness of the supporting structures, taking into account the insulation materials used. It should be noted that the building under construction consists of several enclosing structures through which heat leaks. And each of them has its own percentage of heat loss.
- Up to 30% of the total thermal energy goes through the walls.
- Through floors – 10%.
- Through windows and doors – 20%.
- Through the roof - 30%.
Heat loss at home
That is, it turns out that if the thermal conductivity of all fences is incorrectly calculated, then people living in such a house will have to be content with only 10% of the thermal energy that the heating system emits. 90% is, as they say, money thrown away.
“The ideal house should be built from thermal insulation materials, in which 100% of the heat will remain inside. But according to the table of thermal conductivity of materials and insulation materials, you will not find the ideal building material from which such a structure could be erected. Because the porous structure means low load-bearing capacity of the structure. Wood may be an exception, but it is not ideal either.”
A log wall is one of the most insulated
Therefore, when building houses, they try to use different building materials that complement each other in thermal conductivity. In this case, it is very important to correlate the thickness of each element in the overall building structure. In this regard, frame houses https://doma-rsu.ru/ can be considered ideal. It has a wooden base, we can already talk about a warm house, and insulation that is laid between the elements of the frame building. Of course, taking into account the average temperature of the region, it will be necessary to accurately calculate the thickness of the walls and other enclosing elements.
But, as practice shows, the changes being made are not so significant that we can talk about large capital investments.
Construction of a frame house in terms of its insulation
Let's look at several commonly used building materials and compare their thermal conductivity by thickness.
Thermal conductivity of brick: table by variety
PhotoType of brickThermal conductivity, W/m*K
| Ceramic solid | 0,5-0,8 |
| Ceramic slotted | 0,34-0,43 |
| Porous | 0,22 |
| Silicate solid | 0,7-0,8 |
| Silicate slotted | 0,4 |
| Clinker | 0,8-0,9 |
Thermal conductivity of brickwork at a temperature difference of 10°C
Thermal conductivity of wood: table by species
Wood speciesBirchOak across the grainOak along the grainSpruceCedarMapleLarch
| Thermal conductivity, W/m C | 0,15 | 0,2 | 0,4 | 0,11 | 0,095 | 0,19 | 0,13 |
Wood species Linden Fir Cork Pine across the grain Pine along the grain Poplar
| Thermal conductivity, W/m C | 0,15 | 0,15 | 0,045 | 0,15 | 0,4 | 0,17 |
We recommend: Draft regulator for the boiler - how to install, do-it-yourself setup
The thermal conductivity coefficient of balsa wood is the lowest of all wood species. It is cork that is often used as a heat-insulating material when carrying out insulation measures.
Wood has a lower thermal conductivity than concrete and brick
Table of thermal conductivity of concrete
Concrete in its various variations is the most common building material today, although it is not the “warmest”. In construction, a distinction is made between structural and thermal insulating concrete. The former are used to build foundations and critical components of buildings with subsequent insulation, while the latter are used to build walls. Depending on the region, either additional insulation is applied to them or not.
Comparative table of thermal insulation concrete and thermal conductivity of various wall materials
Aerated concrete is considered the most “warm” and durable. Although this is not entirely true. If you compare the structure of foam blocks and aerated concrete, you can see significant differences. In the first, the pores are closed, while in gas silicates, most of them are open, as if “torn.” This is why in windy weather an uninsulated house made of aerated blocks is very cold. The same reason makes such lightweight concrete more susceptible to moisture.
Thermal conductivity of metals: table
This indicator for metals changes with the temperature at which they are used. And here the relationship is this: the higher the temperature, the lower the coefficient. The table shows the metals that are used in the construction industry.
Type of metalSteelCast ironAluminumCopper
| Thermal conductivity, W/m C | 47 | 62 | 236 | 328 |
Now, as for the relationship with temperature.
- Aluminum at a temperature of -100°C has a thermal conductivity of 245 W/m*K. And at a temperature of 0°C – 238. At +100°C – 230, at +700°C – 0.9.
- For copper: at -100°C –405, at 0°C – 385, at +100°C – 380, and at +700°C – 350.
The thermal conductivity of copper is almost seven times higher than that of steel
Thermal conductivity table for other materials
We will be mainly interested in the table of thermal conductivity of insulating materials. It should be noted that if for metals this parameter depends on temperature, then for insulation it depends on their density. Therefore, the table will display indicators taking into account the density of the material.
Thermal insulation material Density, kg/m³ Thermal conductivity, W/m*K
| Mineral wool (basalt) | 50 | 0,048 |
| 100 | 0,056 | |
| 200 | 0,07 | |
| Glass wool | 155 | 0,041 |
| 200 | 0,044 | |
| Expanded polystyrene | 40 | 0,038 |
| 100 | 0,041 | |
| 150 | 0,05 | |
| Extruded polystyrene foam | 33 | 0,031 |
| Polyurethane foam | 32 | 0,023 |
| 40 | 0,029 | |
| 60 | 0,035 | |
| 80 | 0,041 |
And a table of thermal insulation properties of building materials. The main ones have already been discussed; let us designate those that are not included in the tables and that belong to the category of frequently used ones.
Building material Density, kg/m³ Thermal conductivity, W/m*K
| Concrete | 2400 | 1,51 |
| Reinforced concrete | 2500 | 1,69 |
| Expanded clay concrete | 500 | 0,14 |
| Expanded clay concrete | 1800 | 0,66 |
| Foam concrete | 300 | 0,08 |
| Foam glass | 400 | 0,11 |
What is the thermal conductivity coefficient of the air gap?
In construction, windproof air layers are often used, which only increase the heat conductivity of the entire building. Also, such vents are necessary to remove moisture outside. Particular attention is paid to the design of such layers in foam concrete buildings for various purposes. Such layers also have their own thermal conductivity coefficient depending on their thickness.
Table of heat conductivity of air layers
An excerpt characterizing Thermal Conductivity
“La balance y est... [The balance is established...] A German is threshing a loaf of bread on the butt, comme dit le proverbe, [as the proverb says],” Shinshin said, shifting the amber to the other side of his mouth and winked at the count. The Count burst out laughing. Other guests, seeing that Shinshin was talking, came up to listen. Berg, not noticing either ridicule or indifference, continued to talk about how by transferring to the guard he had already won a rank in front of his comrades in the corps, how in wartime a company commander can be killed, and he, remaining senior in the company, can very easily be company commander, and how everyone in the regiment loves him, and how his daddy is pleased with him. Berg apparently enjoyed telling all this, and did not seem to suspect that other people might also have their own interests. But everything he told was so sweetly sedate, the naivety of his young egoism was so obvious that he disarmed his listeners. - Well, father, you will be in action in both the infantry and the cavalry; “This is what I predict for you,” said Shinshin, patting him on the shoulder and lowering his legs from the ottoman. Berg smiled happily. The Count, followed by the guests, went into the living room. There was that time before a dinner party when the assembled guests do not begin a long conversation in anticipation of the call for appetizers, but at the same time consider it necessary to move and not remain silent in order to show that they are not at all impatient to sit down at the table. The owners glance at the door and occasionally glance at each other. From these glances, guests try to guess who or what else they are waiting for: an important relative who is late, or food that is not yet ripe. Pierre arrived just before dinner and sat awkwardly in the middle of the living room on the first available chair, blocking everyone's path. The Countess wanted to force him to speak, but he naively looked through his glasses around him, as if looking for someone, and answered all the Countess’s questions in monosyllables. He was shy and alone did not notice it. Most of the guests, who knew his story with the bear, looked curiously at this big, fat and humble man, wondering how such a bumpkin and modest man could do such a thing to a policeman. -Have you arrived recently? - the countess asked him. “Oui, madame,” he answered, looking around. -Have you seen my husband? - Non, madame. [No, madam.] - He smiled completely inappropriately. – You, it seems, were recently in Paris? I think it's very interesting. – Very interesting.. The Countess exchanged glances with Anna Mikhailovna. Anna Mikhailovna realized that she was being asked to occupy this young man, and, sitting down next to him, began to talk about her father; but just like the countess, he answered her only in monosyllables. The guests were all busy with each other. Les Razoumovsky... ca a ete charmant... Vous etes bien bonne... La comtesse Apraksine... [The Razoumovskys... It was amazing... You are very kind... Countess Apraksina...] was heard from all sides. The Countess got up and went into the hall. - Marya Dmitrievna? – her voice was heard from the hall. “She’s the one,” a rough female voice was heard in response, and after that Marya Dmitrievna entered the room. All the young ladies and even the ladies, with the exception of the oldest ones, stood up. Marya Dmitrievna stopped at the door and, from the height of her corpulent body, holding high her fifty-year-old head with gray curls, looked around at the guests and, as if rolling up, slowly straightened the wide sleeves of her dress. Marya Dmitrievna always spoke Russian. “Dear birthday girl with the children,” she said in her loud, thick voice, suppressing all other sounds. “What, you old sinner,” she turned to the count, who was kissing her hand, “tea, are you bored in Moscow?” Is there anywhere to run the dogs? What should we do, father, this is how these birds will grow up...” She pointed to the girls. - Whether you want it or not, you have to look for suitors. - Well, what, my Cossack? (Marya Dmitrievna called Natasha a Cossack) - she said, caressing Natasha with her hand, who approached her hand without fear and cheerfully. – I know that the potion is a girl, but I love her. She took out pear-shaped yakhon earrings from her huge reticule and, giving them to Natasha, who was beaming and blushing for her birthday, immediately turned away from her and turned to Pierre. - Eh, eh! kind! “Come here,” she said in a feignedly quiet and thin voice. - Come on, my dear... And she menacingly rolled up her sleeves even higher. Pierre approached, naively looking at her through his glasses. - Come, come, my dear! I was the only one who told your father the truth when he had a chance, but God commands it to you. She paused. Everyone was silent, waiting for what would happen, and feeling that there was only a preface. - Good, nothing to say! good boy!... The father is lying on his bed, and he is amusing himself, putting the policeman on a bear. It's a shame, father, it's a shame! It would be better to go to war. She turned away and offered her hand to the count, who could hardly restrain himself from laughing. - Well, come to the table, I have tea, is it time? - said Marya Dmitrievna. The count walked ahead with Marya Dmitrievna; then the countess, who was led by a hussar colonel, the right person with whom Nikolai was supposed to catch up with the regiment. Anna Mikhailovna - with Shinshin. Berg shook hands with Vera. A smiling Julie Karagina went with Nikolai to the table. Behind them came other couples, stretching across the entire hall, and behind them, one by one, were children, tutors and governesses. The waiters began to stir, the chairs rattled, music began to play in the choir, and the guests took their seats. The sounds of the count's home music were replaced by the sounds of knives and forks, the chatter of guests, and the quiet steps of waiters. At one end of the table the countess sat at the head. On the right is Marya Dmitrievna, on the left is Anna Mikhailovna and other guests. At the other end sat the count, on the left the hussar colonel, on the right Shinshin and other male guests. On one side of the long table are older young people: Vera next to Berg, Pierre next to Boris; on the other hand - children, tutors and governesses. From behind the crystal, bottles and vases of fruit, the Count looked at his wife and her tall cap with blue ribbons and diligently poured wine for his neighbors, not forgetting himself. The countess also, from behind the pineapples, not forgetting her duties as a housewife, cast significant glances at her husband, whose bald head and face, it seemed to her, were more sharply different from his gray hair in their redness. There was a steady babble on the ladies' end; in the men's room, voices were heard louder and louder, especially the hussar colonel, who ate and drank so much, blushing more and more, that the count was already setting him up as an example to the other guests. Berg, with a gentle smile, spoke to Vera that love is not an earthly, but a heavenly feeling. Boris named his new friend Pierre the guests at the table and exchanged glances with Natasha, who was sitting opposite him. Pierre spoke little, looked at new faces and ate a lot. Starting from two soups, from which he chose a la tortue, [turtle,] and kulebyaki and to hazel grouse, he did not miss a single dish and not a single wine, which the butler mysteriously stuck out in a bottle wrapped in a napkin from behind his neighbor’s shoulder, saying or “drey Madeira", or "Hungarian", or "Rhine wine". He placed the first of the four crystal glasses with the count's monogram that stood in front of each device, and drank with pleasure, looking at the guests with an increasingly pleasant expression. Natasha, sitting opposite him, looked at Boris the way thirteen-year-old girls look at a boy with whom they had just kissed for the first time and with whom they are in love. This same look of hers sometimes turned to Pierre, and under the gaze of this funny, lively girl he wanted to laugh himself, not knowing why. Nikolai sat far from Sonya, next to Julie Karagina, and again with the same involuntary smile he spoke to her. Sonya smiled grandly, but apparently was tormented by jealousy: she turned pale, then blushed and listened with all her might to what Nikolai and Julie were saying to each other. The governess looked around restlessly, as if preparing to fight back if anyone decided to offend the children. The German tutor tried to memorize all kinds of dishes, desserts and wines in order to describe everything in detail in a letter to his family in Germany, and was very offended by the fact that the butler, with a bottle wrapped in a napkin, carried him around. The German frowned, tried to show that he did not want to receive this wine, but was offended because no one wanted to understand that he needed the wine not to quench his thirst, not out of greed, but out of conscientious curiosity. At the male end of the table the conversation became more and more animated. The colonel said that the manifesto declaring war had already been published in St. Petersburg and that the copy that he himself had seen had now been delivered by courier to the commander-in-chief.
Disadvantages of the high thermal conductivity of copper and its alloys
Copper has a much higher value than aluminum or brass. But meanwhile, this material has a number of disadvantages that are associated with its positive aspects. The high thermal conductivity of this metal forces the creation of special conditions for its processing. That is, copper billets must be heated more accurately than steel. In addition, there is often pre- or auxiliary heating before starting treatment. We must not forget that pipes made of copper imply that careful thermal insulation will be carried out. This is especially true for those cases when the heating supply system is assembled from these pipes. This significantly increases the cost of installation work. Certain difficulties arise when using gas welding. To get the job done, a more powerful tool is required. Sometimes, to process copper with a thickness of 8 - 10 mm, it may be necessary to use two or even three torches. In this case, one of them welds the copper pipe, and the rest are busy heating it. In addition, working with copper requires more consumables.
Working with copper requires the use of specialized tools. For example, when cutting parts made of bronze or brass with a thickness of 150 mm, you will need a cutter that can work with steel with a large amount of chrome. If it is used for processing copper, then the maximum thickness will not exceed 50 mm.
Why is thermal insulation needed?
The relevance of thermal insulation is as follows:
- Keeps you warm in winter and cool in summer.
Heat loss through the walls of a typical multi-storey residential building is 30-40% . To reduce heat loss, special thermal insulation materials are needed. The use of electric heaters in winter contributes to additional energy costs. It is more profitable to compensate for these costs by using high-quality thermal insulation material, which ensures heat retention in winter and coolness in the summer heat. At the same time, the cost of cooling the room with air conditioning will also be minimized.
- Increasing the durability of building structures.
In the case of industrial buildings using a metal frame, insulation helps protect the metal surface from corrosion, which is the most detrimental defect for this type of structure. And the service life of a brick building is determined by the number of freeze/thaw cycles. The impact of these cycles is perceived by the insulation, because the dew point is located in the thermal insulation material, and not in the wall material.
Such insulation allows you to increase the service life of the building many times.
- Noise insulation.
Protection against increasing noise levels is achieved by using such noise-absorbing materials (thick mattresses, sound-reflecting wall panels).
- Increasing the usable area of buildings.
The use of a thermal insulation system makes it possible to reduce the thickness of external walls, while increasing the internal area of the building.