Compared to conventional zinc plating, a zinc-aluminum (ZA) alloy coating called Galfan gives the steel increased corrosion resistance and formability.
Galfan coating differs from conventional zinc coating by its increased resistance to corrosion. Therefore, it can be used to extend the life of steel products or in place of conventional zinc coating, as Galfan's thinner coating improves the product's weldability and formability.
Galfan's zinc-aluminum coating is easily recognizable by its bright metallic, slightly honeycombed surface. The eutectic alloy, composed of approximately 95% zinc and 5% aluminum, has excellent adhesive properties, and its laminar microstructure provides the ductility necessary for deep drawing. Galfan coating is significantly superior to conventional zinc coatings in terms of formability.
Galfan's zinc-aluminum coating is applied to both sides using a continuous hot-dip process. Galfan coating provides protection against corrosion of steel even in exposed areas, including, for example, cutting edges or areas where the coating may be damaged (scratches, impacts, etc.). The extremely low coefficient of friction and the strong bond of the coating to the steel prevent it from peeling, so complete protection against corrosion extends to areas subject to strong mechanical stress during the molding process.
SSAB offers Galfan-coated steel in a variety of thicknesses, qualities and surface finishes to suit different applications.
Do-it-yourself cold galvanizing - 8 rules for high-quality application
- What is needed to prepare the surface and apply the composition
- Application conditions
- Metal surface cleaning
- Degreasing
- Preparation of working staff
- Application
- General recommendations for application
To protect metal structures and products from corrosion, you do not need to contact specialists, disassemble them and transport them somewhere, or spend a lot of money, effort and time. You just need to buy a cold galvanizing compound, apply it yourself and forget about corrosion for 25 years. Here we will tell you where to buy cold galvanizing and how to apply it, spending a minimum of time and money.
A little about the method
Cold galvanizing of metal structures is the application of a composition with a high (92-96%) zinc content to the prepared metal surface. The method allows you to apply the composition on your own, without the involvement of specialists, and directly at the site of operation of the structures. The result is a durable coating that lasts up to 25 years without renewal.
What do you need?
To prepare the metal surface and apply the composition you need:
1.Sanding machine, if not, then a wire brush or coarse sandpaper
– this is necessary to remove old coating or already formed rust. The grain size of the grinding wheel or sandpaper should be from 5 to 6 according to GOST 3647-71 or from 180 to 220 according to the European standard FEPA.
2. Solvent:
solvent, xylene, solvent-ur or any suitable composition for cold galvanizing. The solvent is necessary for degreasing the surface, diluting the composition to a more liquid consistency and cleaning tools. The amount of solvent depends on the scope of your application job.
3. Construction mixer or drill with a special attachment
, or any object suitable for mixing paint in a jar - it is necessary to thoroughly mix the composition so that the coating lays evenly and without lumps.
4. Tool for application, you can choose: brush, roller, spray gun, airless or air sprayer
– whatever is more convenient for you to apply.
If you have small parts with hard-to-reach areas for application, then it makes sense to apply the composition by immersion. Then you need a convenient plastic container of such a size and depth that your products can completely fit there.
5. Individual protection means:
gloves, a respirator, preferably a protective suit with a hood to avoid getting dirty, as the coating will be very durable.
6. Composition for cold galvanizing
, in the quantity you need.
Conditions for application and subsequent operation may differ for each cold galvanizing composition. Be sure to read the instructions included with the material.
Advice!
The instructions and descriptions of some cold galvanizing compounds indicate that they allow application directly to a rusty and unprepared surface. This is true, but we still recommend that you carefully prepare the surface and remove any remaining rust and old coatings. This will allow the coating to last longer - up to 25 years without updating.
Application conditions
Application temperature from -35°С to +35°С Air humidity 70-98% Outdoors or in a well-ventilated area
Detailed instructions for applying the cold galvanizing composition
1. Cleaning the metal surface
- All traces of dirt must be removed from the metal surface. This can be done using simple, household detergents.
- If the steel is new and there is tightly adhered scale, it is necessary to carry out abrasive blasting cleaning to degree 2 according to GOST 9-402.
- If the metal surface has old coating or rust, they are removed using a grinding machine, brush or sandpaper.
Cleaning can be done well and quickly using water under a pressure of 10-20 MPa.
- If the steel has already been galvanized, then the old coating must also be removed. Here you cannot do without water under a pressure of 10-20 MPa.
- Paint is removed from metal mechanically, chemically or with water under a pressure of 175-275 MPa, whichever is more convenient for you.
- After removing old coatings and dirt, it is necessary to thoroughly dust the surface, not skipping any areas. To do this, it is better to use a cleaning device with compressed air, which must be clean and dry and comply with GOST 9.010-80.
- After cleaning work, the metal surface must be degreased.
- To degrease metal, solvents are used: xylene, solvent or special solvents of the same brand as the composition for cold galvanizing.
- If the metal structure is processed outdoors, then the finished composition must be applied no later than 12 hours after cleaning and degreasing the surface. If they are processed indoors, then no later than 48 hours later. Otherwise, cleaning and degreasing will have to be done again.
3. Preparation of working composition
- Most cold galvanizing compounds are one-component and immediately ready for use. If the composition is two-component, then it must be mixed in accordance with the instructions attached to it.
- It is necessary to open the jar and mix thoroughly until smooth, preferably mechanically.
- If you apply with a spray gun or mechanical spray, you may need to dilute the composition. For dilution, petroleum, coal solvent, xylene or a special solvent of the same brand as the composition are also used.
- The composition is diluted by no more than 5-10% of the total mass. After dilution, as well as every 30 minutes, the composition must be thoroughly mixed again to avoid settling of zinc powder and uneven coating.
- After preparation, the composition is applied to the prepared surface, outdoors - no later than 12 hours, indoors - no later than 48 hours.
- We recommend roughening the surface with sandpaper before application - the adhesion of the composition to the metal will be stronger.
- Application with a brush: It is recommended to choose a brush made of natural bristles. At the same time, it must be cleaned of various contaminants and dust. Apply like regular paint.
- Application with a roller: you need a roller made of material that is resistant to organic solvents. It is important that the roller is cleaned of dust and various contaminants, as well as previously used paints and varnishes. We apply the composition in the same way as regular paint.
- Application using a pneumatic sprayer: the sprayer must be cleaned of dirt, as well as of previously used paints and varnishes. The diameter of the nozzle used is 2.0-3.0 mm. The pressure in the sprayer is 2-3 bar (0.2-0.3 MPa). Try to spray as evenly as possible.
- Application by airless spraying: the equipment used must be cleaned of dirt, as well as of previously used paints and varnishes. The diameter of the nozzle used is 0.38-0.63 mm or 0.015-0.025 inches. The pressure in the sprayer is 80-120 bar (8-12 MPa).
- Application by dipping: immerse the part in a jar or special container with a composition for cold galvanizing. Avoid dipping your hands. The part should be completely submerged, wait 5-10 seconds. After making sure that the composition has reached all hidden cavities and hard-to-reach places, remove the part. Then it is recommended to hang the part until it dries completely and evenly.
- Application from an aerosol can: when applying, the can should be held vertically with the valve up at a distance of 25-30 cm from the surface to be coated. Apply the aerosol at ambient temperatures from +5°C to +40°C. After finishing work, clean the valve: turn the cylinder over and press the nozzle until clean gas begins to come out. Remove any remaining composition on the valve with a swab soaked in a solvent (solvent, xylene).
Corrosion resistance of aluminum coated through a zincate sublayer.
You can read about the study of the corrosion resistance of aluminum with chemical nickel coating through a zincate sublayer in the article.
The thickness and structure of the zinc layer, obtained by immersion or in some other way, affect not only the adhesion strength of aluminum to the coating, but also the corrosion resistance of aluminum with a particular galvanic coating. This coating in relation to aluminum is always cathodic in its electrochemical nature, and the zinc layer is anodic in relation to both the aluminum base and the galvanic coating. This situation determines the specific course of the corrosion process of aluminum subjected to zincate treatment and galvanic coating.
While the corrosion products of steel products bearing cathodic galvanic coatings are rust, which can be removed in one way or another, corrosion of aluminum subjected to zincate treatment and subsequent electroplating usually manifests itself in blistering and peeling of the coating (as the corrosion products zinc are bulky). A diagram of such corrosion is shown in Figure 5.
Thus, Figure 5 (a) shows a cross-section of an aluminum coated sample, in which a recess is made, allowing the environment access to the base metal (alloy), the zinc layer and the galvanic coating. Figure (b) shows the protective effect of zinc in relation to the base metal and to the galvanic coating. This protective action continues until the zinc has dissolved to a certain depth. After this, the aluminum begins to dissolve (c) and the zinc layer is exposed, as a result of which it will again protect both the aluminum base and the galvanic coating (d) from corrosion. In the future, a similar cycle is repeated.
Figure 5 — Scheme of corrosion of an aluminum part coated through a zincate sublayer.
Based on this mechanism of the corrosion process subjected to zincate treatment and galvanic coating of aluminum, we can be guided by the following basic principles:
- With a thinner zinc film, aluminum resists corrosion better, since the lateral permeability of zinc cannot extend deeply: the narrower the gap, the smaller the dissipation ability.
- Those aluminum alloys that themselves have less electronegative potentials resist corrosion better after electroplating. For example, alloys such as duralumin resist corrosion better than unalloyed aluminum due to the smaller potential difference between the base and the galvanic coating.
- Large gaps (pores) in the galvanic coating are less dangerous than small ones, since in the latter case small anodic areas are exposed, functioning as protectors that protect the galvanic coating from corrosion. As a result, rapid corrosion occurs, which leads to swelling and peeling of the sediment. Particularly dangerous are small cracks that appear in more stressed galvanic coatings obtained from electrolytes containing organic brightening agents, which must be used with extreme caution when electroplating aluminum.
- Any modified zincate process that slows down the rate of zinc dissolution during the anodic reaction is beneficial.
One of the main provisions that determine the widespread use of the zincate method for preparing the surface of aluminum is the proximity of the electrochemical potentials of aluminum and zinc in an alkaline environment. As stated above, the corrosion resistance of galvanized aluminum alloys is greater, the thinner the layer of zinc displaced at the time of immersion. Obviously, the smaller the potential difference between the aluminum alloy and zinc, the faster the process of displacing the latter from the alkaline solution will stop, the denser and thinner the zinc film will be. Consequently, the potential difference between a particular aluminum alloy and zinc in an alkaline environment can serve as a criterion for judging the behavior of aluminum alloys during zincate processing and after galvanic coating.
Bengston determined the weight of the zinc film obtained on various aluminum alloys during their zincate processing, and found that the heaviest films are formed on cold-worked AMts and AMg alloys, and the lightest on alloys such as duralumin (Figure 6).
Figure 6 - Weight of the zinc film obtained on various aluminum alloys during their zincate processing.
The potential difference between aluminum and zinc, and therefore the thickness of the zinc film, is also determined by the conditions of preliminary preparation of the aluminum surface. This difference, which is greatest after degreasing aluminum with organic solvents, is significantly smaller after degreasing in an alkaline solution and clarification in nitric acid. Figure 7 shows the effect of the type and duration of pretreatment on the Al-Zn potential difference.
Technology for galvanizing at home
Home page » Metalworking - galvanizing » Galvanic galvanizing
Galvanic galvanizing in production conditions. Photo PKF Color
Galvanizing metal using the galvanization method is a useful and interesting process. quite easy to do it at home , especially in situations where it’s more rational to do everything yourself and save money at the same time. Before starting work, it is important to understand the essence of the process .
When is it more profitable to do it yourself than to order a service?
Considering that the galvanic galvanizing method is expensive, in some cases it is reasonable to create a small chemical laboratory at home and do everything yourself. You can use the method at home :
Small parts after zinc treatment. Photo EnergoStal
- for small metal parts , the size of which will allow them to be immersed in a container with a zinc solution;
- for decorating metal surfaces;
- treat the most vulnerable areas of the car body or small areas of other metal structures.
Painting a galvanized car body
How to paint a galvanized car body? This is a question many car owners ask. After applying the last layer of zinc, you must wait 24 hours for the solution to harden and the binder to evaporate, but be careful. It is recommended to wash the part with a solution of baking soda; in extreme cases, you can also use water to completely remove the acid from the element. Carry out the painting procedure in the usual way, and for this you need to apply a layer of primer, perform its finishing or fine polishing and evenly apply a layer of paintwork.
What you will need
For the galvanic galvanizing method at home, you will need the following devices and substances:
- current source. This could be a charger or a car battery. Its parameters should be in the range from 2 to 6 A and from 6 to 12 V;
- zinc sulfate – 200 g;
- ammonium or magnesium sulfate – 50 g;
Reference. Performers have the opportunity to purchase equipment for galvanic galvanizing by contacting employees of the companies that are presented in the section “Where to buy equipment (on credit, leasing).”
- sodium acetate – 15 g;
- water – 1 l.;
- container into which the parts will be immersed;
- wires for connecting to a power source.
Important. During work, be sure to use protective equipment (respirator, rubber gloves and exhaust device).
Questions - Answers
Since the main focus of this site is informational and educational
, The Site Council decided not to create a “Forum” section, which, as is well known, often turns into an unprofessional and, often, illiterate discussion club.
To receive qualified
answers to questions and for consultations, a section
“QUESTIONS - ANSWERS”
, to which you can send letters by
All questions will be forwarded to leading Russian specialists in the field of electroplating and surface treatment
and then questions and answers will be placed in the same section.
Tip: By clicking the "forward" link, you can view the questions in chronological order. Using the “back” link, you can scroll through the questions in reverse chronological order, starting with the newest ones.
Popular about electroplating and electroplating
- Chrome plating at home
- How to organize electroplating of parts in an auto service station
- Are batteries harmful and what are the consequences of electrolyte evaporation?
- Service life of galvanic coatings
- Electroplating at home (in the garage)
- Is it dangerous to live in a house built on the site of a galvanizing shop?
Economic and legal issues
- Certification of electroplating production according to ISO
- Is certification of galvanic equipment required?
- Pricing in electroplating
- How to reduce fines for exceeding maximum permissible concentrations
- The question of the mandatory nature of REACH and other European regulations in Russia
- Occupational safety and health Interindustry rules on labor protection when applying metal coatings
- Manual loading and unloading of large and heavy products into baths
Processes of electrodeposition of metals and alloys
- Galvanizing newProblems with additives (emulsifier or brightener) in the weakly acidic ammonium-free galvanizing electrolyte
- newWhite coating on zinc coating
- Hydrogenation of thin-walled parts in zincate electrolyte
- Dark spots on the zinc coating of complex profile products
- Cracking and peeling of zinc coating with paintwork from steel on parts with a screw connection
- Differences in the properties of zinc coatings made from electrolytes with additives from different manufacturers
- Treatment of zinc coating before painting
- Porosity during galvanizing of TsAM
- Why is a zinc generator better than anodes or baskets in a galvanizing bath?
- Galvanizing of hardened nitro-carburized steels in zincate electrolyte
- Increasing the rate of dissolution of zinc anodes
- Question about the composition of ammonia electrolyte for galvanizing and passivation bath
- Anodic film, does it interfere with the alkaline galvanizing process?
- Zinc coatings on steel at high temperatures
- Permissible volumetric current density for zincate electrolyte
- Peeling of zinc coating during rolling process
- Problems of bright galvanizing with Colzinc
- Black dots on zincate electrolyte coating
- Selection of brightening agent and type of passivation for bright galvanizing
- Do matte and shiny zinc coatings differ in corrosion properties?
- Overheating of the galvanizing bath
- Comparison of zinc coatings
- Selecting an electrolyte for galvanizing window fittings
- Application of ammonia-free galvanizing electrolyte without brighteners
- Thermal diffusion galvanizing of springs
- Prevent heating of alkaline galvanizing electrolyte
- Galvanizing of parts that have previously undergone nitrocarburization
- Drum galvanizing at high current density
- Potassium chloride galvanizing
- Dark spots during potassium-chlorine galvanizing of bolts on hangers
- Galvanizing of contaminated threaded products
- Problem with galvanizing in ammonia electrolyte
- Problems with galvanizing in a drum
- Questions about galvanizing in cyanide-free alkaline electrolytes
- Dewatering of thin steel discs
- newAbout the causes of turbidity in nickel coating
- How to preserve the color of copper?
- newOperation of tin-nickel electrolyte
- Applying gold with a nickel sublayer to parts made of MD40 alloy
- Cyanide brass plating of TsAM alloys: coating color
- newPitting in cobalt hydrometallurgy
- About the corrosion resistance of copper-nickel-chrome coating and options for its replacement
Chemical coatings
- newColor of chemically deposited nickel coating after heat treatment
- newFiltration of electroless nickel plating solution
- Replacing chemical nickel with electrolytic nickel for soldering
- Conductive coating, resistant to marine environments
- Heating an electroless nickel plating solution using a steam jacket
- About the solderability of chemically deposited nickel
- Heating the electroless nickel plating solution
- Hard nickel plating
- Is it possible to coat part of an aluminum part with chemical nickel?
- About replacing enameled buckets in the electroless nickel plating process
- “Boiling” of the electroless nickel plating solution
- The role of lead sulfide in the electroless nickel plating process
- Deterioration of solderability of parts with chemical nickel-phosphorus coating
- Local overheating of the electroless nickel plating solution
- Anodic protection of titanium nickel plating baths
- Chemical metallization - a question from the Society of Novice Electroplaters
Surface treatment
- Pre-treatment Degreasing AMG
- Titanium etching
- Grinding copper parts with electrocorundum
- Degreasing of parts made of different materials in one solution
- Electrical steel pickling
- Preparation of brass parts before nickel plating
- Steel wire pickling
- Iron precipitate and its permissible concentration in the clarification bath
- Descaling
- About the time between galvanizing and chromating zinc parts
- Colorless passivation after galvanizing
- Temperature of surface preparation with phosphating solution with KFA-8
- Cold oxidation of steel
- newChoice between An.Ox.black. and Chem.Ox.black.
Anodes and anodic processes
- Anode for nickel plating of the inner surface of the cylinder
- Depolarized Nickel Anodes
- Cathode material when anodizing aluminum
- Anodic film, does it interfere with the alkaline galvanizing process?
- Passivation of anodes during nickel plating
- Location of anodes during galvanizing in drums
- Anodic area during galvanizing using titanium baskets
- Anodes for galvanizing in cyanide-free alkaline electrolytes
- Selection of nickel anodes for nickel plating from sulfamate electrolyte
- The influence of temperature and quenching time on the service life of titanium anodes ORTA and ORTA-I (iridium)
- Features of the anodic process in various galvanizing electrolytes
Corrosion protection
- On the possibility of replacing cadmium coatings with zinc-lamella or zinc-nickel alloy
- Corrosion of nickel-plated steel parts during storage
- How to Remove Greenery from a Gilded Bronze Chandelier
- Protecting silver items from tarnishing
- Color change of "colorless" passive film upon drying
- Is Dacromet suitable for protecting fasteners before embedding in concrete?
- Corrosion protection of the diesel locomotive water system pipeline
- Protection of the walls of the internal channels of the cooling system from corrosion
- Question about the universal protective coating “METAS-anticorrosive”
- Adhesion of Dacromet 500 LC coating and compatibility with epoxy paints
- Corrosion resistance of parts in climatic conditions UHL 5
- Corrosion resistance and wear resistance of zinc coatings compared to nickel
- Methodology for determining the corrosion resistance of zinc coating after passivation
- Service life of galvanic zinc coating in alkaline environment
- On the durability of nickel coatings on copper when working in transformer oil
- Dacromet coating, its corrosion properties and the possibility of use in the tropics
- Measuring the degree of corrosion of phosphated and painted surfaces
- Protection of steel products in various climatic conditions
- Protection against atmospheric corrosion. Comparison of galvanizing, anodizing and chrome plating
Electrotype
- Electroplating technology of silver and silver alloys
- What is needed to organize an artistic electroplating workshop?
Removal of electroplating
- Electrochemical removal of chrome plating
- Permissible density of sulfuric acid in solution for removing nickel coating
- Sandblasting removal of galvanic coating
- Removing nickel coating from copper-steel assemblies
- Isolation of a porous surface during chemical removal of nickel plating
- Dewatering tempering of steel after removing the chrome coating
- Removing nickel from a multilayer copper-nickel coating from TsAM zinc alloy parts
- Mechanical and chemical ways to remove chromium
Ecology of galvanic production
- Water consumption issues Calculation of water consumption for washing by spraying parts above the bath
- Washing scheme after chrome plating
- Calculation of water consumption for flushing using a two-stage scheme
- How to prepare water for an evaporator to avoid scale
- The question of drainless technology of galvanic processes with a closed cycle of use of wash waters
- Vacuum wastewater evaporation method
- Removal of excess carbonates from cyanide electrolytes prepared on the basis of potassium salts
- Disposal of hydrochloric acid after removing zinc coating with it
- How ferritization of galvanic sludge affects the hazard class of waste
Electroplating equipment
- newSelection of material for the heat exchanger
- newCombination of ventilation from the oil bath and galvanic bath
- Insulation of suspension devices with galvanic tape or plastisol
- Zirconium heating elements for heating electrolytes of nickel sulfate plating
- Possibility of using aluminum cathode rods for chrome plating
- Selecting a heater and material for an electroless nickel plating bath
- Thermal insulating floats made of polypropylene
- How to properly design an aluminum hard anodizing bath
- Voltage of heating elements in galvanic baths
- What temperature can PVC and polypropylene equipment withstand?
- Drums with partial immersion in electrolyte
- Criteria for choosing suspension design
- Materials for lining chrome plating baths
- AC Rectifiers Which rectifier is needed for the aluminum anodizing process
- Special requirements for current sources used for chrome plating
Quality control
- Methods for determining antimony in silver electrolyte
- Quantitative determination of copper impurity in bright nickel plating electrolyte
- Determination of chloride ions in chromic acid anodizing electrolyte
- Purification of cadmium sulfate electrolyte
- Measuring the concentration of gold and nickel with the Corian-3 analyzer
- Analysis of electrolyte for chemical oxidation of aluminum for chromium and fluoride content
- Express methods for determining phosphorus compounds in electrolyte for chemical nickel plating
- Methods for analyzing gold plating electrolyte
- Analysis of nickel plating electrolyte for iron content
- Determination of the presence of hexavalent chromium compounds in conversion films on zinc
- Method for determining the current efficiency of zinc
- Express methods for determining hexa- and trivalent chromium in chromic acid electrolytes for chrome plating
General engineering issues
- Terminology Is anodic film an electroplating?
- Difference between Chemical Ox.fluorine and Chemical Ox.e coatings
- Coating Chemical Ox.e
- English terms for electroplating
- What is the difference between a metal coating shop and a site?
- Digital designation of types of electroplating
- newChoice between An.Ox.black. and Chem.Ox.black.
- newPowder Coating Remover
Information and its sources
- newGOST for nickel-phosphorus coating
- Methods for determining potassium silicofluoride
- Copper plating modes on reverse current
- Error in analyzes of electrolytes for gilding, silvering, and palladizing
- Question about advanced training courses in galvanizing
- Mechanical and corrosion properties of zinc coatings according to M.A. Schluger and in the reference book of Yu.D. Hamburg
- Search for Zinclamel Coating Services
- Erroneous data in report abstracts
- About the article on “trivalent chromium plating” in the magazine “World of Electroplating”
- About the book “Physico-chemical foundations of electrochemistry” by Lukomsky and Hamburg
- Hard Anodizing Processes
- Search for companies using galvanic production
- At which enterprises over the past 5-10 years have they created new galvanic workshops or carried out reconstruction?
- Electrolysis at very high current densities in stationary and pulsed modes
Galvanizing a small part
The car part to be processed is prepared accordingly. Traces of corrosion and old paintwork are removed. When cleaning, do not use aggressive paint removers. If you still cannot do without them, then the part should be treated with an aqueous solution of soda.
Next, the required amount of zinc-containing solution and the appropriate container are prepared. The negative terminal of the battery is connected to the part, and it is immersed in the solution. The capacitance itself is connected to the positive terminal and voltage is applied. For successful galvanizing of a small part, a voltage of 12 V and a current of about 1 A will be sufficient. The result of the treatment should be a part with a uniform gray coating. Next, the element is removed and thoroughly rinsed in an aqueous soda solution.
How and what to do
Galvanizing can be divided into the following stages :
- First of all, the solution is prepared in a glass container of a suitable size;
- the zinc solution is heated to a temperature ranging from 18 to 25 degrees;
- after this, the anodes are immersed in the solution, which are connected to the positive on the current source;
- At a safe distance from the zinc solution, fix the product that needs to be galvanized, and connect the “negative” wire (cathode) to it;
- after this, the galvanization process should begin, in which a zinc composition is deposited on the product through the negative wire.
Important. The current should not exceed 1 A, otherwise the coating will be of poor quality.
Galvanizing at home
Process theory.
Galvanic galvanizing (zinc plating) is the process of depositing metal cations on the anode. The reaction takes place in an electrolytic bath under the influence of electric current.
Any solution of zinc salt can serve as an electrolyte. The most common in everyday life is a solution of ZnCl (zinc chloride) and HCl (hydrochloric acid), which is called soldering acid. You can also obtain a ZnSO4 solution by etching zinc in a H2SO4 solution (sulfuric acid is a battery electrolyte). When etching zinc, you should be extremely careful, since the reaction occurs with the release of heat and explosive hydrogen H2.
General preparation of aluminum surface.
The issue of degreasing metals (including aluminum) is discussed in the article.
The process of etching aluminum has its own characteristics. When etching a number of chemical processes occur on the surface of aluminum. First, the dissolution of aluminum oxide in the cleanest places with the release of hydrogen and the formation of aluminates. Next, the dissolution of aluminum metal will occur with the formation of the same products, and the dissolution of the metal on the protrusions will proceed faster than in the recesses, due to which the surface will be leveled from large scratches. At the same time, surface etching will increase and micro-roughness will increase.
It is important that the etching of aluminum that has intermetallic compounds on the surface will proceed primarily along them (see article, paragraph 5.2).
When loading parts into the pickling bath, several degreasing factors also begin to work:
- Sodium hydroxide saponifies fats.
- The surfactant improves the wettability of parts and reduces the surface tension of the fat film.
- Actively released hydrogen mechanically tears the oil and fat film into the solution volume, where it is exposed to the action of the first two factors.
In some cases, during the general preparation of the surface of aluminum parts, it is justified to use a gentle etching solution that does not have a degreasing effect and is used for processing products with welded unsealed seams.
At the end of etching, a loose layer of sludge remains on the surface of the parts, consisting of products insoluble in alkali. The exact composition of the sludge depends on the alloying additives included in the aluminum alloys. Sludge removal occurs during the clarification operation. The composition of the solutions is different for wrought and cast alloys and is determined by the composition of the slurry. Wrought alloys are brightened in nitric acid, while aluminum foundry sludge, rich in silicon, does not dissolve in it. It requires adding hydrofluoric acid to the solution. Nitric acid does not act on aluminum, passivating it. Largely due to this, thin oxide layers remain on aluminum, preventing further metallization, but not preventing oxidation.
When is it more profitable to turn to professionals than to do it yourself?
To enhance the strength of a metal product using galvanic galvanization, the power of home equipment is not suitable . Therefore, if there is a need to strengthen metal products, give them strength characteristics or restore them , then in such a situation it is recommended to use the services of professionals. With the help of galvanic galvanizing, you can protect and restore the car body, but it is also better to entrust this to the professionals of the enterprises from the section “Where to order galvanic galvanizing.”
Reference. Alternatives to the galvanic method are hot galvanizing, cold galvanizing, gas-thermal, thermal diffusion. Each method has its own advantages and disadvantages.
Having started galvanizing at home, it is important to follow all the recommendations and subtleties of the process . Even though it is quite simple, it is important to be precise and do everything carefully. High-quality zinc coating of metal requires a competent approach.
Electroplating: Anodized aluminum
Aluminum is a great material, and here's why: it's easy to work with, strong and lightweight. Today it is used for many purposes. But aluminum has a drawback - it oxidizes very quickly. This is where we need anodizing technology, which we will discuss in detail below.
An oxide film usually appears on anodized aluminum. The main advantage of the anodizing process is that the treated area of the material subsequently becomes more durable. It can be said that, as with any electroplating process, the material is protected from corrosion by applying an additional coating.
Why is the anodizing process so important?
Essentially, it is a chemical process. If you understand how corrosion of metal products spreads, then you know for sure that oxygen is a powerful oxidizing agent for metal. As a result of its interaction with the material, oxides are formed, and then the process of destruction begins. Anodizing a metal strengthens the oxide film. And, of course, not the primary, but also important point - the appearance of aluminum becomes “tidier” and is easier to paint further.
The benefits of using anodized aluminum
The processed material is widely used in industry, in floodlights, heating reflectors, etc.
Warm anodic oxidation
Used for painting metal. The technology is quite simple, but here’s the catch - as a result, the surface of the aluminum profile is easily destroyed. The coating is also easy to wipe off, just rub it with your hand... Additional epoxy coating will be required.
Cold anodizing
A great way to anodize at home. It happens as follows: the layer on the inside of the metal becomes stronger due to dissolution on the outside. The main thing is to keep the temperature low. Using the cold anodizing method, you will have to abandon organic dyes.
Getting started with the anodized coating of aluminum
First, let's prepare the aluminum bathtubs. The main thing is to take care of thermal insulation so that the mixture does not heat up. It is important! Next, we make a cathode from lead sheets; the cathode area should be twice the surface area of the material that we are processing.
Next, we degrease the sample from all possible contaminants and grind the part, after which we dip it in alkali. At this time, micropores begin to form on the surface of the material, and the surface becomes denser.
Now let's move on to chemical processing. We place an electrolyte (a solution of sulfur or chromium, oxalic or sulfosalicylic acid) into the bath. We recommend that you use chromic or oxalic acid. If you are anodizing aluminum at home, it is best to use a soda solution.
Pores of different diameters appeared on the part. They need to be closed for strength. To do this, lower the part into hot water, and then place it in a cold solution. There is no need to fix it only if the aluminum is coated with paint after anodizing, because it already closes the pores.
Aluminum galvanizing: These mistakes are best avoided!
First of all, be sure to take care of your safety. Wear gloves, goggles and special protective clothing. You work with chemistry, and this is fraught with disastrous consequences.
An important point is that the temperature should not be too low. High resistance will make it more difficult to maintain current density. If you work at home, the ideal temperature would be -10 degrees Celsius. At high temperatures, expect the opposite effect - the color will turn out cloudy and the anodic coating will be weak.
Suitable coating density
We answer right away - it is best when the density of the anodic coating is 2-2.2 A per dm2. This will protect you from possible mistakes. Do not increase the current power! This will cause damage to the part.
By the way, about current power
If the metal part is in good contact with the pendant, it will provide optimal amperage. Good contact can be established using an aluminum pin clamp, then the electrode will be tightly attached to the part.
Main types of anodizing
Now we invite you to consider the types of anodizing in more detail. Here are the main ones:
- Solid
A mixture of acids for the use of anodic coating in industry (in the automotive industry, for example). The main quality of hard anodizing is durability.
- Microarc
The metal surface is oxidized and is excellent for further adhesion.
- Colored
In other words, this is the painting of a material (using adsorption, or integral/interference/electrolytic coloring).
Now you understand the process of anodizing aluminum in a little more detail. We have also tried to explain how electroplating is done at home. However, for anodizing services for aluminum and its alloys, we recommend that you contact companies verified by PromMarket specialists. You learn from mistakes, but it is much safer to entrust galvanizing to a professional with knowledge of his craft and experience. Good luck!
Materials for performing the galvanizing procedure
Aerosol for cold galvanizing “Zinconol” To carry out the cold galvanizing process, it is necessary to use zinc-containing primer, which is sold in specialized stores. The most popular means for cold galvanizing are:
The most common galvanizing agent chosen by motorists is Zinconol.
- Aerosol "Zinconol";
- Corrosion protection agent “Zinkor”;
- Primer "Cinotan";
- Paint for galvanizing metal.
The most common product chosen by motorists is Zinconol, since it has all the necessary technical characteristics to protect metal. Another product that will help restore the protective functions of the metal is the composition for cold galvanizing “Galvanol”.
Galvanic processing of aluminum. Theoretical part
Galvanic processing of aluminum is a process of anodic oxidation. During galvanic treatment, an oxide film is formed on the surface of aluminum. At the same time, the outer surface of this oxide film dissolves in the galvanic solution. If the solubility of the oxide decreases due to a change in any process parameters (for example, a drop in temperature), then the oxide film becomes thinner. In other words, the anodic treatment does not use galvanic treatment, but anodization - the creation of a thin film of oxides on the surface of aluminum.
You can understand the conditions necessary for galvanic processing of aluminum by comparing it with chemical and electrochemical surface treatment of aluminum, which includes:
Etching
Anodizing to create a thin protective film of oxide in solutions that do not have solvent properties
Anodizing to create a thin oxide film in solutions with solvent properties
Chemical treatment
Adjustable operating parameters include:
- Voltage used
- Temperature
- Solubility of aluminum oxide in solution
- Oxidizing capacity of solution
All these factors are interconnected.
These two types of processing - chemical and galvanic - involving the oxidation of aluminum, are the basis for the main aluminum processing processes, and will be discussed in more detail below. The purpose of these processes is to build up an oxide film of constant thickness, which would prevent the entry of air onto the surface of the metal and thereby protect it from corrosion. This is achieved by using acid solutions that have a weak dissolving effect on the film, under the influence of which the latter becomes sufficiently porous to provide access for the film-forming medium to the metal. In anodic oxidation, if the dissolution ratio is low enough (for example, boric acid or ammonium phosphate electrolytes), then film growth soon stops, which in turn makes it possible to create a dielectric film at high voltage, for example, for subsequent use in capacitors. If the dissolution coefficient is not so low (as, for example, in sulfuric acid solutions used in industrial anodizing processes), then the thickness of the oxide film increases in proportion to the anodization time. However, an infinite increase in the thickness of the oxide film is impossible, since it ultimately stops when the rate of oxide growth on the aluminum layer is balanced by the dissolution coefficient in the area of contact between the oxide and the solution.
However, at constant voltage there is a certain maximum value to which the maximum film thickness increases with increasing dissolution rate. This value is reached when the film buildup ratio decreases and balances the dissolution ratio, causing film dissolution to occur faster than oxide buildup. Under such conditions, it becomes possible to create durable films of extreme thickness, which in turn makes it possible to carry out galvanic processing. As we will see, the actual dissolution rate and the film thickness at which galvanic treatment occurs can have very different values. When using some electrolyte solutions, the treatment is carried out with films of sufficient thickness to protect against corrosion, but a durable film may be only a few molecules thick. For example, as a result of galvanic processing according to the Brital method (sodium carbonate and trisodium phosphate) and the Alzack method (fluoroborate solutions, etc.), a film of Al2O3 is formed of sufficient thickness to obtain interference colors and a high degree of protection. In other processes, especially in concentrated acid solutions, the possibility of creating strong films has long been questioned, but today it has been proven and allows us to answer questions that could not previously be solved using theories based on diffusion layers.
The fact that metals are coated with a durable film during certain electrical and chemical processing processes has been demonstrated by Hoare et al. by testing the wettability of mercury. The presence of a strong film during galvanic processing of aluminum was also demonstrated by Raub and Baba, who relied on its capacitance for sizing. Using an electron microscope, it was also shown that this film has a porous structure, like any anodic oxide coating, and the pore sizes and density depend on the electrolysis conditions. When the current was interrupted, the film dissolved and the capacity increased again. Fujinara also studied the appearance of surface films during galvanic processing of 99.9% aluminum using various electrolytes and proved that the film consists of a barrier layer with a thickness of 0.2-2.0 micrometers and a porous layer with a thickness of up to 0.5 micrometers, the thickness of each layer being proportional constant tension. It is therefore not surprising that the current waveform has an impact on the plating treatment. Steiner, who studied this phenomenon, described both the positive and negative effects of alternating current. It is known that the Brital process cannot occur efficiently when using single-phase rectified current. Hoar, Mears and Rothwell stated that the oxide contains impurity anions through which cations can pass and produce anodic gloss. In the presence of chlorides or other aggressive ions, disruption of the field permeability in some areas stimulates the entry of anions into the film in the weakest places, which leads to ruptures and pitting. The higher current density helps restore a strong film and return to shiny surface conditions.
Therefore, as expected, the film formed under galvanic treatment conditions does not differ in quality from that produced during industrial anodization. Lichtenberger-Baiza and Hollo claim that depending on the base metal, after galvanic treatment in solutions of phosphoric acid or butyl alcohol, this film will have a thickness of 0.1-0.2 micrometers, and the creation of thicker films is possible on purer metals. Processing time does not affect thickness, and the film is based on a barrier type structure.
Lichtenberger-Baisa, who used the Hunter method to determine the thickness of barrier-type anodic oxide coatings, obtained thickness values of 0.5-5.0 micrometers during galvanic treatment of aluminum with phosphorus-chromium sulfur, phosphorochromic and hydrofluorosulfuric acids, as well as phosphoric electrolytes acids or butyl alcohols. As with industrial anodizing, the thickness of the barrier layer increases when using a higher current density. However, as the concentration of phosphoric acid increases, a higher current density is required to obtain the same thickness of the barrier layer.
In addition, both anodic oxide films formed in anodizing electrolytes and the film obtained by galvanic treatment consist of hexagonal cells, and the diameter of both cells and pores increases with increasing electrical potential. At a voltage of 30-35 volts, the cell diameter is approximately 10 micrometers, and the pore diameter is 6 micrometers. The thickness of the barrier layer formed on pure and ultra-pure aluminum in solutions of phosphoric acid and butyl alcohols does not exceed 0.5 micrometers, and on Al-Cu-Mg alloys - less than 0.1 micrometers.
However, on films produced with industrial anodizing electrolytes, on electrically treated aluminum, a porous film can be discerned above the barrier layer of electrochemically treated aluminum. Chemical analysis of the film produced on 99.99% aluminum shows that it consists of 79.9% Al2O3 and 7.3% AlPO4, plus 0.064% Fe and 0.31% Si.
Advantages and disadvantages of galvanizing body elements
Galvanization can hardly be called a panacea, but the advantages of this method of anti-corrosion treatment are obvious:
- a thin layer of zinc well protects ferrous metal from exposure to oxygen, moisture, and salts, preventing the spread of corrosion areas;
- galvanization, depending on the application method, can last on body parts for 5-25 years;
- Coating iron parts with zinc is more reliable and much cheaper than using tin.
Unfortunately, such protection is not eternal - on average, the zinc layer thins by 1-5 microns every year. Disadvantages include higher cost compared to treatment with a special primer. But if you do it yourself, the procedure will not be so burdensome financially.
Degreasing, pickling and activation of aluminum and alloys
Degreasing
The process of degreasing the surface of metal parts is carried out, as a rule, when these parts have just been processed (ground or polished) and there is no rust, scale or other foreign products on their surface. Using degreasing, oil and grease films are removed from the surface of parts. For this purpose, aqueous solutions of certain chemical reagents are used, although organic solvents can also be used for this.
| Components | Compositions for degreasing aluminum and alloys | ||
| №1 | №2 | №3 | |
| Soda ash, g/l | 5-10 | 50-60 | 20-25 |
| Trisodium phosphate, g/l | 5-10 | 50-60 | 20-25 |
| Emulsifier OP-7 (OP-10), g/l | 15-20 | — | 5-7 |
| Liquid glass, g/l | 25-50 | 20-30 | — |
| Temperature | 50-60 | 50-60 | 70-80 |
| Time of processing | 3-5 | 3-5 | 10-20 |
Etching
Pickling (as a preparatory operation) allows you to remove contaminants (rust, scale and other corrosion products) from metal parts that are firmly adhered to their surface. The main purpose of etching is to remove corrosion products; in this case, the base metal should not be etched. To prevent metal etching, special additives are added to the solutions. Good results are obtained with the use of small amounts of hexamethylenetetramine (urotropine).
| Compositions for etching aluminum and its alloys (g/l) | ||||
| Components | №1 | №2 | №3 | № 4 |
| Nitric acid 1.4 g/cm | — | 35…40 | — | — |
| sodium chloride | — | — | — | 30 |
| Sodium hydroxide | 50…100 | — | 25…35 | 150 |
| soda ash | — | — | 20…30 | — |
| Temperature, C | 40…60 | 18…25 | 40…60 | 60 |
| Processing time, sec | 5… 10 | 3…5 | 0,5…2,0 | 15…20 |
Decapitation (activation)
Activation is the removal from the surface of parts of the thinnest layers of oxides that form during washing and in intervals between operations. When activated, the top layer of metal is lightly etched, which ensures strong adhesion of the applied coating to the base metal. Activation is carried out immediately before applying galvanic coatings.
| Composition for pickling aluminum and its alloys | |
| Components | №1 |
| Nitric acid | 10-15% solution |
| Temperature, C | 20 |
| Processing time, sec | 5…15 |
OPTION 2
Chemical preparation of products before obtaining oxide coatings includes the operations of degreasing, etching, and metal brightening . Depending on the nature and degree of surface contamination, organic solvents or aqueous solutions are used.
When choosing the composition of aqueous degreasing solutions, in order to reduce their aggressive effect on the metal, the content of caustic alkali is reduced in comparison with that adopted for degreasing ferrous metals, and sometimes the concentration of sodium carbonate in the solution is increased. Improving the quality and accelerating the degreasing process is facilitated by the introduction of surfactant additives, especially synthanol, as well as an increase in the concentration of phosphates.
To degrease products made of aluminum and its alloys, you can use solutions of the following compositions (g/l):
1) sodium carbonate - 10-20, trisodium phosphate - 5-50, tripolyphosphate - 3-5, synthanol DS-10 - 8-10; 2) sodium carbonate - 15-20, trisodium phosphate - 25-30, synthanol DS-10 - 3-4; 3) sodium hydroxide - 10-15, trisodium phosphate - 50-60, sodium metasilicate - 20-30.
Degreasing is carried out at a temperature of 60-80 °C. Solutions 1, 2 are used to treat polished parts, solution 3 - for heavily soiled parts.
Already during the degreasing process, since it involves the use of alkaline solutions, more or less etching of the metal surface occurs. Therefore, a special etching operation is included in the technological process not so much to remove corrosion products, as when etching ferrous metals, but to obtain a certain surface texture.
A fine-grained silver surface is obtained by treating aluminum in a solution containing 120-150 g/l sodium hydroxide and 25-35 g/l sodium chloride at a temperature of 60-70 °C. To matt aluminum and its deformable alloys, you can use a solution containing 50-200 g/l of a mixture consisting (mass fraction, %) of 56 sodium nitrate, 44 sodium hydroxide; at a temperature of 40-60 °C.
The processing of aluminum and its alloys, even in weakly concentrated alkaline solutions, is accompanied by the release of sludge of insoluble components - copper, silicon, manganese - on their surface. To remove them, a lightening operation . Technical aluminum and its deformable alloys with magnesium, manganese, copper, types AMg, AMts, D16, V95 are clarified in 30-50% nitric acid or in a solution containing 100 g/l chromic anhydride and 5 g/l sulfuric acid. Casting alloys such as silumin are processed in a solution containing 250-300 g/l nitric acid and 8-10 g/l hydrofluoric acid.
To brighten AL9 and ALU alloys after alkaline etching, use a solution consisting of 0.7 l of nitric acid (1.41), 0.3 l of hydrofluoric acid (40%), 15 g/l chromic anhydride. This preparation is especially effective for products that are then subjected to chemical oxidation.
The processed products are loaded into the oxidation bath on hanging fixtures made of aluminum, duralumin or titanium. When reusing aluminum hangers, the oxide film must first be removed . To do this, they are poisoned in a hot 10% solution of caustic alkali. It is also advisable to use for this purpose a solution containing 20 g/l chromic anhydride and 35 ml/l phosphoric acid (1.52). Processing is carried out at a temperature of 90-100°C.
Poor-quality oxide coatings can be removed from the surface of products by treating them in solutions of the following compositions:
1) chromic anhydride - 20 g/l, phosphoric acid (density 1.5) - 35 ml/l; 2) nitric acid (density 1.34) - 50-55 ml/l, hydrofluoric acid (40%) -4.5 ml/l.
In solution 1 at a temperature of 85-100°C, it takes 10-30 minutes to remove the oxide film, in solution 2 at room temperature 30-50 minutes. There is almost no change in the dimensions of parts during etching.
Gas-thermal galvanizing method
Galvanizing using this algorithm is carried out by spraying. This method is good only in cases where it is necessary to process large surfaces and in large batches. Otherwise, the profitability of this method will be questionable. Galvanization using the gas-thermal method is carried out by spraying powdered zinc or zinc in the form of a thin wire. Zinc is supplied to the metal in molten and sprayed form. It is used in cases where the dimensions of the product do not allow it to be placed in a bath of liquid zinc, most often these are large-sized metal structures. After galvanizing, the metal surface is a porous coating, and the pores are most often filled with paint and varnish material. Due to its strength properties and resistance to aggressive environments, this combined coating is considered one of the most durable and can preserve metal even in salt water for 25-35 years.
Selecting an aluminum alloy for anodizing.
A special feature of the anodizing process is the fact that the oxide coating is not applied externally, but is formed from the top layer of aluminum or its alloy. Consequently, not only aluminum itself, but also alloying components are involved in the process. In this case, they can: • Dissolve and pass into the electrolyte. Such elements are, for example, copper, iron, magnesium, which form intermetallic compounds with aluminum.
• Oxidize and integrate into the structure of the oxide film, changing its color, physical and chemical properties. Titanium has this property.
• Remain indifferent, releasing in the form of sludge as the oxide film moves deeper into the part. Such sludge can either be captured by the oxide film or form a poorly adherent smear layer on the surface of the part. A typical example is silicon, which is present in abundance in foundry alloys.
The choice of material for anodizing is based on these features of the behavior of impurities in aluminum alloys:
• Technically pure aluminum is best anodized.
• The fewer alloying additives in the alloy, the thicker and more decorative the oxide film can be obtained. When decorative anodizing, the aluminum alloy should not contain more than (%): 8 - zinc; 7 - magnesium; 3 - silicon; 2 - copper; 0.8 - manganese, 0.5 - iron; 0.3 - titanium; 0.3 - chromium. In this case, the total alloying should not be more than 8%. The lightest coatings are obtained on technically pure aluminum, fairly light ones on AMg alloys, dark gray with some yellowness on D16. Alloy AD31 (6063) is relatively difficult to etch; sometimes on its surface after anodizing, variations in color are clearly visible in places of contamination, even after good degreasing of the parts
• Aluminum alloys containing copper, magnesium, iron, manganese become rougher after anodizing, at the same time they are better filled with dyes (the resulting color is more saturated) and adhere better to paint coatings.
• The color of the anodic film is influenced by the structure of the metal. In places with mechanical damage, the process goes faster and, accordingly, the color of the film may be darker. Such “foci” can be scratches and cut points, and on a sandblasted surface the film can generally turn out chaotically spotted. It often happens that after waterjet cutting of a sheet part during anodizing, the outer surface turns out to be much lighter than the cut site, which, if you do not know the specifics of the process, can be mistaken for a defect.
• When anodizing, it is desirable to have a technological platform on the parts for mounting on hangers (threaded hole, unthreaded hole, tail, stud, etc.). You cannot simply hang parts on hooks (as with metallization) - the hook itself will be anodized, not the part. It is necessary to create such a tight electrical contact that electrolyte cannot get under it. Therefore, heavy parts (from 1 kg with 1 current supply) are better anodized, because Their weight alone creates good contact with the suspension. Lightweight parts, especially hardware, always require the design and manufacture of special titanium tooling. Without it, anodizing is either impossible or proceeds with the idle speed of the bath up to 90%.
• Bulk anodizing (similar to galvanizing) is not possible.
• Thin and light aluminum plates cannot be coated in a band - deformation of the part will necessarily occur at the point of contact.
• It is not advisable to anodize a part consisting of different aluminum alloys. Different alloys have different structure, thermal conductivity, and chemical resistance. All this can lead to different anodizing modes on different parts of the same part, redistribution of electric current over the surface and irreversible defects.
• If necessary, parts should be heat treated before anodizing, because It is undesirable to heat the anode film above 100°C.
• Thick anodic films (19.5-25 microns per 1 hour of process) are formed on technically pure aluminum and alloys AD1, D16, V95, D20, AMg, AMts, AL2, AL8. Thin (7.6-8.5 microns per 1 hour of process) - on D1, AL7.
Thermal diffusion method
Galvanizing a metal using this method involves the penetration of zinc atoms into the structure of a metal product to form an iron-zinc alloy with a complex structure. The entire process takes place at very high temperatures. At temperatures above 2600 degrees, zinc goes into a gaseous state. The closed space where metal products are located is filled with zinc-containing powder. The method is suitable for applying a layer whose thickness exceeds 15 microns. The resulting protective coating can be compared in terms of stability to a layer applied using the hot method. Due to the need to create certain conditions, the method is not suitable for independent implementation.
Types of galvanizing metal surfaces
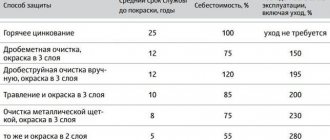
Currently, there are several methods for applying a thin layer of zinc to a metal surface. All of them are used in industry with varying degrees of success:
- cold/hot galvanizing;
- gas-thermal galvanizing;
- galvanic method;
- thermal diffusion treatment.
Each of these methods uses different physical and chemical technological processes to apply zinc coating. Let's look at them in more detail.
Hot galvanizing
It is considered one of the most effective galvanizing methods. Zinc applied in this way lasts much longer, protecting metal parts for the longest possible time. Among the main disadvantages of this method, one can highlight the environmental component - the use of a chemical method of applying a layer of zinc contributes to environmental pollution. The hot-dip galvanizing method involves the use of the following technological chain:
- at the preparatory stage, metal surfaces to be galvanized are degreased, after which they are etched with acid;
- after such treatment, the part is washed in water and dried thoroughly;
- then the product is placed in a container filled with molten zinc.
In addition to harm to the environment, hot-dip galvanizing has other disadvantages:
- such processing requires the use of expensive special equipment;
- There are also restrictions on the dimensions of the parts being processed (it will not be possible to process the entire car body).
Cold galvanizing
This method of anti-corrosion treatment is one of the simplest. Its essence is to paint a metal surface with a special paint that contains zinc. It does not matter what method is used to apply the coloring composition: it can be a roller, a spray gun or a brush. The combination of powder paint and a spray gun allows galvanizing of any parts, regardless of their size, which is why it is often used as an alternative to hot-dip galvanizing. In particular, the entire vehicle body is galvanized using this method. Cold galvanizing also allows for the re-application of a protective layer on a previously galvanized surface.
Galvanic processing method
Galvanizing a car body using the galvanic method involves treating metal surfaces susceptible to corrosion through electrochemical action. The advantages of this method over cold galvanizing are the possibility of applying a more uniform and very thin layer of zinc. Its essence is to place the part in a tank filled with water, in which a zinc plate is placed. After applying electricity, the process of diffusion of zinc atoms begins, which are transferred from the plate to the workpiece, forming a very thin protective layer. As in the case of hot-dip galvanizing, galvanic treatment is considered an environmentally very dirty method, and also quite expensive - in particular, a lot of money is spent on waste water treatment.
Gas thermal galvanizing
This method is not perfect, but with its help it is possible to process very large parts. It involves blowing the surfaces with a gas stream containing zinc. This is how the bodies of large vehicles are galvanized, but in this case the zinc layer is applied unevenly, requiring additional painting of the body parts. Of course, this method is characterized by the use of specialized equipment, so it is certainly not applicable in garage conditions.
Thermal diffusion galvanizing
As the name suggests, this processing method is based on the use of high temperatures. The physics of the process consists in the splitting of zinc into atoms at temperatures exceeding 2500-2600 degrees, as a result of which the processed parts are covered with a layer of zinc, the thickness of which can be as large as desired without loss of quality. To use thermal diffusion galvanizing, the use of a special sealed heat chamber is required. A layer of zinc powder of a certain thickness is first applied to the metal surface, after which the chamber is heated to the specified temperatures, the powder melts and is adsorbed onto the surface of the ferrous metal. The quality of such galvanizing is quite high, and does not cause any harm to the environment.
The main disadvantage of using this method is its high cost, so it is used exclusively in industrial conditions.
Zincate method for preparing aluminum surfaces.
3.1 Kinetics of the process.
When aluminum is immersed in a solution of sodium zincate, the oxide film dissolves and metallic zinc is displaced from the solution. From this point of view, the zincate method resembles other chemical methods for preparing aluminum in solutions of acids containing salts of heavy metals. The difference between this method is that zinc in a highly alkaline environment is in the form of complex ions and its potential is much more negative than the potentials of iron or nickel in simple solutions, for example, in solutions of chlorides. Therefore, the potential difference in a highly alkaline environment between zinc and aluminum is significantly less than between iron or nickel and aluminum in solutions of simple salts.
When immersing aluminum in a solution of sodium zincate, you can expect to obtain a thinner, more uniform and dense film, which should promote strong adhesion to the galvanic coating without visible traces of etching of the base metal, which is extremely important when applying protective and decorative coatings.
The behavior of aluminum in a solution of sodium zincate is considered as an electrochemical process. Aluminum dissolves at the anodic sites, zinc is released at the cathodes:
at the anode areas:
2H+ + 2e → 2H → H2
Since, however, in a highly alkaline environment the concentration of hydrogen ions is extremely low, and the overvoltage of hydrogen on zinc is quite large, the last reaction can be neglected. In practice, the formation of a zinc film on aluminum is not accompanied by visible evolution of hydrogen.
The quantitative relationships between dissolving aluminum and displaced zinc were determined by Bengston. He found that when ordinary (commercial) aluminum is immersed in a zincate solution, the following ratios are observed (in mg/cm2):
| Aluminum dissolves: | 0,017 |
| Zinc is actually released: | 0,057 |
| Theoretically, zinc is released: | 0,062 |
3.2 Structure of zincate films on aluminum.
It will be shown below that the thickness and structure of the zinc film depend on many factors. The interaction of aluminum with solutions of sodium zincate is of not only theoretical but also practical interest, since it determines the adhesion strength of aluminum to galvanic coating.
Based on the lattice parameters of aluminum (4.04 A) and zinc (2.66 A), Ballach and Gardham believed that by replacing two aluminum atoms with three zinc atoms, a molecular layer of zinc could be formed on the (110) plane without significant distortion. And on the (111) plane a hexagonal lattice with noticeable distortion can form.
Figure 1 shows a bevel cut of aluminum after immersion in sodium zincate solution for 3 minutes and subsequent coating with copper. The cut was made at an angle of 5° to the surface in order to make the precipitation more visual.
a) b)
Figure 1 - An oblique cut of aluminum after 3 minutes of immersion in a solution of sodium zincate and subsequent coating with copper: a - zinc film obtained from a dilute solution of zincate, b - from a concentrated solution.
From these figures, as well as from electron microscopy studies, Bailey concluded that a dilute zincate solution containing 5 g/l ZnO and 45 g/l NaOH produced thick, coarse-crystalline, dendritic zinc precipitates, while more a concentrated solution containing 50 g/l ZnO and 262.5 g/l NaOH produces thin, dense and finely crystalline precipitates. It was found that the zinc film released from concentrated solutions is covered with a thin film of zinc hydroxide (approximately 100 A thick) and, according to Bailey, who studied 14 zincate solutions, such a film promotes strong adhesion of aluminum to the galvanic coating. Based on theoretical calculations, Bailey established that the grain size of the zinc film obtained from concentrated solutions is approximately 100 times smaller than the size of grains obtained from dilute solutions.
Bailey investigated the effect of the concentration of the main components of a zincate solution on the adhesion strength of aluminum to a 50-μm-thick nickel coating. In this case, the following conventions were adopted: if the coating peeled off from the base when heated, the adhesion was considered weak; if the coating was peeled off by a chisel blow, the adhesion was considered average, and if it was impossible to separate the coating from the base by any means, the adhesion was characterized as strong.
The experimental results are shown in Figure 2.
Figure 2 - Effect of the concentration of the main components of the zincate solution on the adhesion strength of aluminum to a nickel coating 50 microns thick, where a - treatment was carried out for 0.5 minutes, b - 3.0 minutes, c - 5.0 minutes.
For a given zinc content in the solution, the thickness of the displaced zinc film is smaller, the higher the alkali content in the solution. This dependence is quite understandable if we take into account the fact that alkali is a complexing agent in a zincate solution and that the strength of complex ions increases with the concentration of alkali. From Figure 3 it can be seen that the highest rate of film growth is observed in the first 15 seconds, then the film grows slowly at any alkali content in the solution.
Figure 3 - Effect of exposure time of a part in a zincate solution on the weight of the film.
The film growth rate increases as the temperature increases, as can be seen in Figure 4.
Figure 4 - Effect of the temperature of the zincate solution on the weight of the film
In a solution containing 520 g/l NaOH and 100 g/l ZnO, it is possible to ensure strong adhesion of the coating to the base after a one-minute exposure at a temperature of 85 ° C, while after a three-minute exposure the adhesion is poor. At temperatures below 20°C, the reaction rate slows down and at 6°C, a 20-minute exposure is required to ensure strong adhesion. Usually the zincate solution is kept at room temperature.
Is it difficult to check this yourself?
It is impossible to visually determine whether a machine or part of it is galvanized. But there are a few points that will help you figure this out:
- When purchasing a car, carefully inspect the body. 99% of the time you will find a couple of scratches. If the place where the paintwork was chipped simply darkened and there was no rust, the car or part of it was galvanized.
- Use the vehicle's VIN code, which contains technical characteristics, availability of protection and application method.
- Warranty card information. It says here about guarantees of anti-corrosion protection of the body. If a period of 5 to 10 years is indicated, this means cold galvanizing, 10-20 years - the galvanic method, more than 20 - the hot method.
- If the documents indicate that the car is galvanized, but the cost of the car is low, perhaps we are talking about either partial processing or the cold method.
If the machine has full anti-corrosion protection, the technical documentation indicates “full galvanization”.
Source