Most often, metals have crystal lattices of the following types: Body-centered cubic or abbreviated bcc (lead, tungsten) 9 atoms; Face-centered cubic (fcc) (silver, gold) 14 atoms; hexagonal close-packed (hcp) (magnesium, zinc). FCC and HCP lattices are more compact than bcc.
All metals are crystalline bodies that have a certain type of crystal lattice, consisting of low-mobility positively charged ions, between which free electrons move (the so-called electron gas
).
This type of structure is called a metallic bond
.
The type of lattice is determined by the shape of an elementary geometric body, the repeated repetition of which along three spatial axes forms the lattice of a given crystalline body.
Metals have relatively complex types of cubic lattices - body-centered (bcc) and face-centered (fcc) cubic lattices.
The basis of the bcc lattice is an elementary cubic cell (Fig. 1.2, b), in which positively charged metal ions are located at the vertices of the cube, and another atom in the center of its volume, i.e. at the intersection of its diagonals. Iron, chromium, vanadium, tungsten, molybdenum and other metals have this type of lattice in certain temperature ranges.
In an fcc lattice (Fig. 1.2, c), the unit cell is a cube with centered faces. Iron, aluminum, copper, nickel, lead and other metals have a similar lattice.
The third common type of close-packed lattices is hexagonal close-packed (HCP, Fig. 1.2, d). The GPU cell consists of spaces spaced from each other by a parameter c
parallel centered hexagonal bases. Three ions (atoms) are located on the middle plane between the bases.
For hexagonal lattices, the ratio of the parameter with
/
a
is always greater than one. Magnesium, zinc, cadmium, beryllium, titanium, etc. have such a lattice.
Didn't find what you were looking for? Use the search:
Best sayings:
Only sleep brings a student closer to the end of the lecture.
And someone else's snoring alienates him. 8588 – | 7405 – or read all.
91.146.8.87 © studopedia.ru Not the author of the materials posted. But it provides free use. Is there a copyright violation? Write to us | Feedback.
Disable adBlock! and refresh the page (F5)
very necessary
One of the most common materials that people have always preferred to work with has been metal. In each era, preference was given to different types of these amazing substances. Thus, the IV-III millennium BC is considered the Chalcolithic, or Copper Age. Later it is replaced by bronze, and then the one that is still relevant today comes into force - iron.
Today it is generally difficult to imagine that it was once possible to do without metal products, because almost everything, from household items, medical instruments to heavy and light equipment, consists of this material or includes individual parts from it. Why did metals manage to gain such popularity? Let’s try to figure out what the features are and how this is inherent in their structure.
General concept of metals
"Chemistry. 9th grade" is a textbook used by schoolchildren. It is here that metals are studied in detail. A large chapter is devoted to the consideration of their physical and chemical properties, because their diversity is extremely great.
Read also: What current to charge AA batteries
It is from this age that it is recommended to give children an idea of these atoms and their properties, because teenagers can already fully appreciate the significance of such knowledge. They see perfectly well that the variety of objects, machines and other things around them is based on a metallic nature.
What is metal? From the point of view of chemistry, these atoms are usually classified as those that have:
- a small number of electrons at the outer level;
- exhibit strong restorative properties;
- have a large atomic radius;
- As simple substances, they have a number of specific physical properties.
The basis of knowledge about these substances can be obtained by considering the atomic-crystalline structure of metals. It is this that explains all the features and properties of these compounds.
In the periodic table, most of the entire table is allocated to metals, because they form all the secondary subgroups and the main ones from the first to the third group. Therefore, their numerical superiority is obvious. The most common are:
All metals have a number of properties that allow them to be combined into one large group of substances. In turn, these properties are explained precisely by the crystalline structure of metals.
Iron, properties of the atom, chemical and physical properties.
Fe 26 Iron
55.845(2) 1s2 2s2 2p6 3s2 3p6 3d6 4s2
Iron is an element of the periodic system of chemical elements of D.I. Mendeleev with atomic number 26. It is located in the 8th group (according to the old classification - a secondary subgroup of the eighth group), the fourth period of the periodic system.
Iron atom and molecule. Iron formula. Structure of the iron atom
Isotopes and modifications of iron
Properties of iron (table): temperature, density, pressure, etc.
Physical properties of iron
Chemical properties of iron. Interaction of iron. Chemical reactions with iron
Getting iron
Application of iron
Table of chemical elements D.I. Mendeleev
Properties of metals
The specific properties of the substances in question include the following.
- Metallic shine. All representatives of simple substances have it, and most have the same silvery-white color. Only a few (gold, copper, alloys) are different.
- Malleability and plasticity - the ability to deform and recover quite easily. It is expressed to different degrees in different representatives.
- Electrical and thermal conductivity are one of the main properties that determine the areas of application of a metal and its alloys.
The crystalline structure of metals and alloys explains the reason for each of the indicated properties and speaks about their severity in each specific representative. If you know the features of such a structure, then you can influence the properties of the sample and adjust it to the desired parameters, which is what people have been doing for many decades.
Crystallization of alloys
The transition of a metal from a liquid to a solid state with the formation of a crystalline structure is called primary crystallization.
The formation of new crystals in a crystalline solid is called secondary crystallization (recrystallization).
The crystallization process consists of two simultaneous processes:
- crystal nucleation;
- linear crystal growth;
Crystals can nucleate spontaneously (spontaneous crystallization) or nucleate and grow on existing ready-made crystallization centers (non-spontaneous crystallization) (Figure 33).
Figure 34 Growth of nucleation centers and crystal growth
Spontaneous crystallization (Fig. 35) is due to the desire of the substance to have a more stable state, characterized by a decrease in the thermodynamic potential G, a characteristic of the free energy of the system. The second law of thermodynamics is that any system always strives to occupy a state such that it has min free energy. The temperature at which the thermodynamic potentials of a substance, both in solid and liquid states, are equal is called the equilibrium temperature (thermodynamic temperature) TG.
Fig.35 Spontaneous crystallization
Thermodynamic potential is determined by:
G = E – TS + РV (according to Helmholtz)
where G is the thermodynamic potential, the free energy of the system,
E – internal energy of the system,
T – thermodynamic temperature
S – entropy (state function: order and disorder associated with translational and oscillatory motion),
РV – work of external forces (pressure on volume)
G = H – TS (according to Gibbs)
where H is enthalpy (E + РV) the sum of the work of internal and external forces.
The difference between the equilibrium ( ТG .) and real ( Тр ) crystallization temperature is called the degree of supercooling ( ΔТ ).
The formation of nuclei is facilitated by fluctuations , i.e. deviation of the energy of groups of atoms in individual zones of a liquid metal from a certain average value.
The appearance of nuclei changes the thermodynamic potential (free energy) of the entire system. On the one hand, during the transition of a liquid to a crystalline state, the thermodynamic potential G decreases, on the other hand, it increases (+) due to the appearance of an interface between the liquid and the crystalline nucleus.
Figure 36 shows how the free energy of the system changes during crystallization.
Kinetics of crystallization . The rate of formation of nuclei formed per unit time per unit volume (1mm-3s-1); growth rate - an increase in the linear dimensions of the growing crystal per unit time (mm/s). Both processes are associated with the movement of atoms and depend on temperature (degree of supercooling Δ T).
Non-spontaneous crystallization (heterogeneous)
Under real conditions, crystallization processes and the nature of the forming structures largely depend on the available ready-made crystallization centers. These centers are:
- refractory particles of non-metallic inclusions;
- oxides;
- intermetallic compounds formed by impurities.
Refining the structure helps improve the mechanical properties of the metal.
Fig. 36 Change in free energy during crystallization
In practice, a technological operation called modification . It consists of introducing modifiers (boron into steel, sodium into aluminum and its alloys). Cooling the metal before pouring to temperatures slightly above the melting point of the metal helps to reduce the grain size.
Crystal Formation
The shape and size of grains formed during crystallization depend on:
- speed and direction of heat removal:
- temperature of liquid metal;
- impurity content.
The structure of the ingot depends on many factors: (Fig. 37)
- quantity and properties of impurities in pure metal;
- the amount of alloying elements in the alloy;
- alloy casting temperature;
- cooling rate during crystallization, etc.
Fig. 37 Diagram of the structure of a metal ingot obtained at different temperatures
The typical structure of an alloy ingot consists of three zones: (Fig. 38)
- small equiaxed crystals on the surface of the ingot, due to the high degree of supercooling;
- columnar crystals, most favorably oriented with respect to heat removal, located normal to the walls of the mold;
- equiaxed large crystals in the middle of the ingot, where the lowest degree of supercooling is observed and no directional heat removal is felt.
A structure consisting of only columnar crystals is called transcrystalline. Found in ingots of very pure metals.
Chemical heterogeneity in individual zones of the ingot is called zonal segregation . It negatively affects the mechanical properties of the alloy. In real alloys, in addition to zonal segregation, there are other types of segregation.
Atomic crystal structure of metals
What is this structure, what is it characterized by? The name itself suggests that all metals are crystals in the solid state, that is, under normal conditions (except for mercury, which is a liquid). What is a crystal?
This is a conventional graphic image constructed by intersecting imaginary lines through the atoms that line up the body. In other words, every metal is made up of atoms. They are located in it not chaotically, but very correctly and consistently. So, if you mentally combine all these particles into one structure, you will get a beautiful image in the form of a regular geometric body of some shape.
This is what is commonly called the crystal lattice of a metal. It is very complex and spatially voluminous, therefore, for simplicity, not all of it is shown, but only a part, an elementary cell. A set of such cells, collected together and reflected in three-dimensional space, forms crystal lattices. Chemistry, physics and metallurgy are sciences that study the structural features of such structures.
The unit cell itself is a set of atoms that are located at a certain distance from each other and coordinate a strictly fixed number of other particles around themselves. It is characterized by packing density, distance between constituent structures, and coordination number. In general, all these parameters are characteristics of the entire crystal, and therefore reflect the properties exhibited by the metal.
Read also: How to burn wood with electric current
There are several types of crystal lattices. They all have one feature in common: the nodes contain atoms, and inside there is a cloud of electron gas, which is formed by the free movement of electrons inside the crystal.
Structure and state of aggregation of substances
There are three states of aggregation: solid, liquid and gas. Each of them assumes a certain arrangement of particles. Below we will describe in more detail how the crystal lattice and the aggregative state of a substance are related in chemistry, but for now we will highlight the general principles.
- If the particles move chaotically, and the distance between them is many times greater than their own sizes, it is a gas . Due to the great distance from each other, the molecules and atoms in such a substance weakly interact with each other.
- If the particles are still arranged randomly, but at a small distance from each other, it is a liquid . In the liquid state of a substance, its molecules and atoms have stronger bonds that are more difficult to break.
- If the particles are collected close to each other and in a certain order, it is a solid . In this state, the connections between them are strongest. Particles can only move within their location and hardly move in space.
Most substances can be in a solid, liquid, or gaseous state, and depending on pressure and temperature, they can easily change from one to another. A typical example is water, which turns into steam when heated and becomes solid ice when cooled.
Types of crystal lattices
Fourteen lattice structure options are usually combined into three main types. They are as follows:
- Body-centered cubic.
- Hexagonal close-packed.
- Face-centered cubic.
The crystal structure of metals was studied only through electron microscopy, when it became possible to obtain high magnification images. And the classification of types of lattices was first given by the French scientist Bravais, by whose name they are sometimes called.
Body-centered lattice
The structure of the crystal lattice of metals of this type is the following structure. This is a cube with eight atoms at its nodes. Another one is located in the center of the free internal space of the cell, which explains the name “body-centered”.
This is one of the options for the simplest structure of the unit cell, and therefore the entire lattice as a whole. The following metals have this type:
- molybdenum;
- vanadium;
- chromium;
- manganese;
- alpha iron;
- beta iron and others.
The main properties of such representatives are a high degree of malleability and ductility, hardness and strength.
Face-centered lattice
The crystal structure of metals having a face-centered cubic lattice is the following structure. This is a cube that includes fourteen atoms. Eight of them form lattice nodes, and another six are located, one on each face.
They have a similar structure:
The main distinctive properties are shine of different colors, lightness, strength, malleability, increased resistance to corrosion.
APPLICATION
Iron is one of the most used metals, accounting for up to 95% of global metallurgical production.
Iron is the main component of steels and cast irons - the most important structural materials.
Iron can be part of alloys based on other metals - for example, nickel. Magnetic iron oxide (magnetite) is an important material in the production of long-term computer memory devices: hard drives, floppy disks, etc.
Ultrafine magnetite powder is used in many black and white laser printers mixed with polymer granules as a toner. This uses both the black color of the magnetite and its ability to stick to the magnetized transfer roller.
The unique ferromagnetic properties of a number of iron-based alloys contribute to their widespread use in electrical engineering for magnetic cores of transformers and electric motors.
Iron(III) chloride (ferric chloride) is used in amateur radio practice for etching printed circuit boards.
Ferrous sulfate heptate (ferrous sulfate) mixed with copper sulfate is used to combat harmful fungi in gardening and construction.
Iron is used as an anode in iron-nickel batteries and iron-air batteries.
Aqueous solutions of ferrous and ferric chlorides, as well as its sulfates, are used as coagulants in the purification processes of natural and waste water in the water treatment of industrial enterprises.
Defects in the crystal structure of metals
However, all types of cells considered may also have natural shortcomings, or so-called defects. This may be due to various reasons: foreign atoms and impurities in metals, external influences, and so on.
Therefore, there is a classification that reflects the defects that crystal lattices may have. Chemistry as a science studies each of them in order to identify the cause and method of elimination so that the properties of the material are not changed. So, the defects are as follows.
- Spot. They come in three main types: vacancies, impurities or dislocated atoms. They lead to deterioration of the magnetic properties of the metal, its electrical and thermal conductivity.
- Linear or dislocation. There are edge and screw ones. They deteriorate the strength and quality of the material.
- Surface defects. Affects the appearance and structure of metals.
Currently, methods have been developed to eliminate defects and obtain pure crystals. However, it is not possible to completely eradicate them; an ideal crystal lattice does not exist.
General structure
Metals are solid substances with a crystalline structure. The exception is mercury, a liquid metal. Crystal lattices are metal atoms ordered in a certain way. Each atom consists of a positively charged nucleus and several negatively charged electrons. Metal atoms don't have enough electrons, so they are ions.
A unit of a crystal lattice is an elementary crystal cell, in the conventional nodes and on the faces of which there are positively charged ions. They are held together by metallic bonds that arise due to the random movement of electrons separated from the atoms (due to which the atoms turned into ions).
Negatively charged electrons keep positively charged electrons at an equal distance, giving the crystal lattice the correct geometric shape. Rice. 1. Metal connection diagram.
The free movement of electrons determines the electrical and thermal conductivity of metals.
The importance of knowledge about the crystalline structure of metals
From the above material, it is obvious that knowledge about the fine structure and structure makes it possible to predict the properties of the material and influence them. And the science of chemistry allows you to do this. The 9th grade of a general education school places emphasis in the learning process on developing in students a clear understanding of the importance of the fundamental logical chain: composition - structure - properties - application.
Read also: What kind of solder to solder microcircuits with?
Information about the crystalline structure of metals very clearly illustrates this dependence and allows the teacher to clearly explain and show children how important it is to know the fine structure in order to correctly and competently use all the properties.
Content:
Definition of crystal lattice
As we know, all material substances can exist in three basic states: liquid, solid, and gaseous. True, there is also a state of plasma, which scientists consider no less than the fourth state of matter, but our article is not about plasma. The solid state of a substance is therefore solid because it has a special crystalline structure, the particles of which are in a certain and clearly defined order, thus creating a crystal lattice. The structure of the crystal lattice consists of repeating identical elementary cells: atoms, molecules, ions, and other elementary particles connected by various nodes.
RESERVES AND PRODUCTION
Iron is one of the most common elements in the solar system, especially on the terrestrial planets, in particular on Earth. A significant part of the iron of the terrestrial planets is located in the cores of the planets, where its content is estimated to be about 90%. The iron content in the earth's crust is 5%, and in the mantle about 12%.
Iron is quite widespread in the earth's crust - it accounts for about 4.1% of the mass of the earth's crust (4th place among all elements, 2nd among metals). In the mantle and earth's crust, iron is concentrated mainly in silicates, while its content is significant in basic and ultrabasic rocks, and low in acidic and intermediate rocks.
A large number of ores and minerals containing iron are known. Of greatest practical importance are red iron ore (hematite, Fe2O3; contains up to 70% Fe), magnetic iron ore (magnetite, FeFe2O4, Fe3O4; contains 72.4% Fe), brown iron ore or limonite (goethite and hydrogoethite, respectively FeOOH and FeOOH nH2O ). Goethite and hydrogoethite are most often found in weathering crusts, forming so-called “iron hats”, the thickness of which reaches several hundred meters. They can also be of sedimentary origin, falling out of colloidal solutions in lakes or coastal areas of the seas. In this case, oolitic, or legume, iron ores are formed. Vivianite Fe3(PO4)2·8H2O is often found in them, forming black elongated crystals and radial aggregates.
The iron content in sea water is 1·10−5-1·10−8%
Industrially, iron is obtained from iron ore, mainly hematite (Fe2O3) and magnetite (FeO Fe2O3).
There are various ways to extract iron from ores. The most common is the domain process.
The first stage of production is the reduction of iron with carbon in a blast furnace at a temperature of 2000 °C. In a blast furnace, carbon in the form of coke, iron ore in the form of agglomerate or pellets, and flux (such as limestone) are fed from above, and are met by a stream of forced hot air from below.
In addition to the blast furnace process, the process of direct iron production is common. In this case, pre-crushed ore is mixed with special clay, forming pellets. The pellets are fired and treated in a shaft furnace with hot methane conversion products, which contain hydrogen. Hydrogen easily reduces iron without contaminating the iron with impurities such as sulfur and phosphorus, which are common impurities in coal. Iron is obtained in solid form and is subsequently melted in electric furnaces. Chemically pure iron is obtained by electrolysis of solutions of its salts.
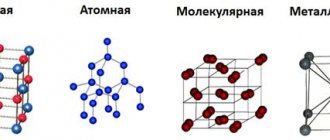
Types of crystal lattices
Depending on the particles of the crystal lattice, there are fourteen types of it, here are the most popular of them:
- Ionic crystal lattice.
- Atomic crystal lattice.
- Molecular crystal lattice.
- Metal crystal lattice.
Next, we will describe in more detail all types of crystal lattice.
Atomic crystal lattice
Substances with an atomic crystal lattice, as a rule, have strong covalent bonds in their nodes consisting of atoms themselves. A covalent bond occurs when two identical atoms share fraternal electrons with each other, thus forming a common pair of electrons for neighboring atoms. Because of this, covalent bonds bind atoms tightly and evenly in a strict order - perhaps this is the most characteristic feature of the structure of the atomic crystal lattice. Chemical elements with similar bonds can boast of their hardness and high melting point. Chemical elements such as diamond, silicon, germanium, and boron have an atomic crystal lattice.
ORIGIN
Origin telluric (terrestrial) iron is rarely found in basaltic lavas (Uifak, Disko Island, off the western coast of Greenland, near Kassel, Germany). At both locations, pyrrhotite (Fe1-xS) and cohenite (Fe3C) are associated with it, which is explained by both the reduction by carbon (including from the host rocks) and the decomposition of carbonyl complexes such as Fe(CO)n. In microscopic grains, it has more than once been established in altered (serpentinized) ultrabasic rocks, also in paragenesis with pyrrhotite, sometimes with magnetite, due to which it arises during reduction reactions. Very rarely found in the oxidation zone of ore deposits, during the formation of swamp ores. Findings have been recorded in sedimentary rocks associated with the reduction of iron compounds with hydrogen and hydrocarbons.
Almost pure iron was found in lunar soil, which is associated with both meteorite falls and magmatic processes. Finally, two classes of meteorites - stony-iron and iron - contain natural iron alloys as a rock-forming component.
Metal crystal lattice
The type of bond of a metal crystal lattice is more flexible and ductile than the ionic one, although in appearance they are very similar. Its distinctive feature is the presence of positively charged cations (metal ions) at lattice sites. Between the nodes live electrons that participate in the creation of the electric field; these electrons are also called electric gas. The presence of such a structure of a metal crystal lattice explains its properties: mechanical strength, heat and electrical conductivity, fusibility.