Technical copper
In the annealed state, it is quite plastic, but has relatively low strength. The chemical composition of technical grades of copper is determined by GOST 859–41. Technical grades are used in the smelting of copper alloys as a charge material. Technical grade M0 is used in the manufacture of high-purity alloys and current conductors. M1 in the manufacture of semi-finished products obtained by rolling, in the production of high-quality bronzes that do not contain tin. M2 is a technical grade used for the production of bronzes for the production of high-quality semi-finished products that are processed under pressure. The MZ technical grade is in demand for semi-finished products that are produced by rolling, the production of standard quality bronzes and other casting alloys, as well as non-critical electrical contacts (like the M2 alloy). Technical grade M4 is used in the smelting of cast bronzes.
Appendix A (for reference). Conformity of grades according to GOST 859–2014, BS EN 1412:1996, ISO 1190−1:1982
Appendix A (reference)
________________ * Access to international and foreign documents mentioned here and further in the text can be obtained by following the link to the website shop.cntd.ru. — Note from the database manufacturer. Table A.1 - Conformity of grades according to GOST 859–2014, BS EN 1412:1996 [1] and ISO 1190−1:1982 [2]
| Copper grade | ||
| GOST 859- | BS EN 1412:1996 and ISO 1190−1:1982 | |
| Designation | European number | |
| M00k | Cu-SATH-1 | CR001A |
| M1k | Cu-CATH-2 | CR002A |
| M00 | Cu-ETP1 | CW003A |
| M0, M1 | Cu-ETP | CW004A |
| M00b | Cu OFE1 | CW009A |
| M0b | CuOF1 | CW007A |
| M1r | Cu-DLP | CW023A |
| M1f | Cu-DHP | CW024A |
Impurities in copper alloys
Impurities contained in copper (and, naturally, interacting with it) are divided into three groups.
Forming solid solutions with copper
Such impurities include aluminum, antimony, nickel, iron, tin, zinc, etc. These additives significantly reduce electrical and thermal conductivity. The grades that are primarily used for the production of conductive elements include M0 and M1. If the copper alloy contains antimony, its hot pressure treatment becomes significantly more difficult.
Impurities that do not dissolve in copper
These include lead, bismuth, etc. Although they do not affect the electrical conductivity of the base metal, such impurities make it difficult to process by pressure.
Impurities that form brittle chemical compounds with copper
This group includes sulfur and oxygen, which reduces the electrical conductivity and strength of the base metal. The sulfur content of the copper alloy greatly facilitates its machinability by cutting.
Copper grades and their applications
Normative references
This standard uses references to the following standards:
GOST 9717.1—82 Copper. Method of spectral analysis using metal standard samples with photoelectric recording of the spectrum GOST 9717.2-82 Copper. Method of spectral analysis using metal standard samples with photographic recording of the spectrum GOST 9717.3-82 Copper. Method of spectral analysis for oxide standard samples GOST 13938.1-78 Copper. Methods for determining copper GOST 13938.2-78 Copper. Methods for determining sulfur GOST 13938.3-78 Copper. Method for determining phosphorus GOST 13938.4-78 Copper. Methods for determining iron GOST 13938.5-78 Copper. Methods for determining zinc GOST 13938.6-78 Copper. Methods for determining nickel GOST 13938.7-78 Copper. Methods for determining lead GOST 13938.8-78 Copper. Methods for determining tin GOST 13938.9-78 Copper. Methods for determining silver GOST 13938.10-78 Copper. Methods for determining antimony GOST 13938.11-78 Copper. Method for determination of arsenic GOST 13938.12-78 Copper. Methods for determining bismuth GOST 13938.13-93 Copper. Methods for determining oxygen GOST 13938.15-88 Copper. Methods for determination of chromium and cadmium GOST 27981.0-88 High purity copper. General requirements for methods of analysis GOST 27981.1-88 High purity copper. Methods of atomic spectral analysis GOST 27981.2-88 High purity copper. Method of chemical-atomic emission analysis GOST 27981.3-88 High-purity copper. Method of emission spectral analysis with photoelectric recording of the spectrum GOST 27981.4-88 High purity copper. Methods of atomic absorption analysis GOST 27981.5-88 High purity copper. Photometric methods of analysis GOST 27981.6-88 High purity copper. Polarographic methods of analysis ST SEV 543-77 Numbers. Recording and rounding rules
Percentage composition
| Percentage composition of material | |||||||||||||
| Copper alloy grades | Fe | Ni | S | Cu | As | Pb | O | Sb | Bi | Sn | P | Zn | Ag |
| M1 | ≤ 0.005 | ≤ 0.0020 | ≤ 0.004 | 99.9 | ≤ 0.0020 | ≤ 0.005 | ≤ 0.05 | ≤ 0.002 | ≤ 0.001 | ≤ 0.002 | ≤ 0.004 | ≤ 0.003 | |
| M 1р | ≤ 0.005 | ≤ 0.0020 | ≤ 0.005 | 99.9 | ≤ 0.0020 | ≤ 0.005 | ≤ 0.01 | ≤ 0.002 | ≤ 0.001 | ≤ 0.002 | from 0.002 to 0.012 | ≤ 0.005 | |
| M 2 | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.7 | ≤ 0.01 | ≤ 0.01 | ≤ 0.07 | ≤ 0.005 | ≤ 0.0020 | ≤ 0.05 | |||
| M 2p | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.7 | ≤ 0.01 | ≤ 0.01 | ≤ 0.01 | ≤ 0.005 | ≤ 0.0020 | ≤ 0.05 | |||
| M 3 | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.5 | ≤ 0.01 | ≤ 0.050 | ≤ 0.08 | ≤ 0.050 | ≤ 0.003 | ≤ 0.05 | |||
| M 3р | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.5 | ≤ 0.05 | ≤ 0.03 | ≤ 0.01 | ≤ 0.05 | ≤ 0.003 | ≤ 0.05 | from 0.005 to 0.06 |
3 Technical requirements
3.1 The chemical composition of copper must correspond to that indicated in tables 1 and 2. When recording and preparing accompanying documentation, it is allowed to indicate the mass fraction of impurities in copper of all grades in grams per ton (parts per million, ppm). The correspondence of copper grades according to this standard and standards [1] and [2] is given in Appendix A.
3.2 The mass fraction of chemical elements not listed in tables 1 and 2 is established by agreement of the parties in accordance with the contract.
3.3 Requirements for the physical properties of copper - electrical resistivity, helical elongation (ability to recrystallize under given heat treatment parameters) and mechanical properties are established in standards for specific types of products and/or as agreed by the parties to the contract.
3.4 The chemical composition of copper, depending on the grade, is determined by
GOST 13938.11, GOST 13938.13, GOST 9717.2, GOST 9717.3, GOST 27981.1,
GOST 27981.2, GOST 27981.5, GOST 27981.6, GOST 31382.
It is allowed to use other measurement techniques (methods), certified in the prescribed manner in accordance with GOST 8.010.
Arbitration methods of analysis indicate specific types of products in the standards.
(Changed edition, Amendment No. 1).
3.5 The results of the analysis of each element are rounded according to the rounding rules established by ST SEV 543, to the number of characters established in tables 1 and 2.
Table 1 - Chemical composition of copper cathode
In percentages
| Chemical element | Mass fraction of element for brands | |||
| M00k | M0k | M1k | ||
| Copper, no less | — | 99,97 | 99,95 | |
| Impurities by group, no more: | ||||
| 1 | Bismuth | 0,00020 | 0,0005 | 0,001 |
| Selenium | 0,00020 | — | — | |
| Tellurium | 0,00020 | — | — | |
| 1st group amount | 0,0003* | — | — | |
| Chromium | — | — | — | |
| Manganese | — | — | — | |
| Antimony | 0,0004 | 0,001 | 0,002 | |
| Cadmium | — | — | — | |
| Arsenic | 0,0005 | 0,001 | 0,002 | |
| Phosphorus | — | 0,001 | 0,002 | |
| 2nd group amount | 0,0015 | — | — | |
| 3 | Lead | 0,0005 | 0,001 | 0,003 |
| 4 | Sulfur | 0,0015 | 0,002 | 0,004 |
| 5 | Tin | — | 0,001 | 0,002 |
| Nickel | — | 0,001 | 0,002 | |
| Iron | 0,0010 | 0,001 | 0,003 | |
| Silicon | — | — | — | |
| Zinc | — | 0,001 | 0,003 | |
| Cobalt | — | — | — | |
| 5th group amount | 0,0020 | — | — | |
| 6 | Silver | 0,0025 | 0,0025 | 0,003 |
| Sum of listed impurities | 0,0065 | — | — | |
| Oxygen, no more | — | 0,015 | 0,02 | |
| ________________ * Including the maximum content of the sum of selenium and tellurium should be no more than 0.00030%. Notes 1 The mass fraction of oxygen for copper grade M00k is established in the contract. 2 The sign “-” means that this element is not standardized. | ||||
Table 1 (Changed edition, Amendment No. 1).
Table 2 - Chemical composition of cast and deformed copper
In percentages
| Copper grade | Mass fraction of element | Method of receipt (for reference) | |||||||||||||
| Copper, no less | Copper + silver, no less | Impurities, no more | |||||||||||||
| Bismuth | Iron | Nickel | Zinc | Tin | Antimony | Arsenic | Lead | Sulfur | Oxygen | Phosphorus | Silver | ||||
| M00b | 99,99 | — | 0,0005 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,0003 | 0,002 | Remelting cathodes in a reducing or inert atmosphere or vacuum |
| M0b | — | 99,97 | 0,001 | 0,004 | 0,002 | 0,003 | 0,002 | 0,002 | 0,002 | 0,003 | 0,003 | 0,001 | 0,002 | — | |
| M1b | — | 99,95 | 0,001 | 0,004 | 0,002 | 0,003 | 0,002 | 0,002 | 0,002 | 0,004 | 0,004 | 0,003 | 0,002 | — | |
| M00 | 99,96 | — | 0,0005 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,002 | 0,03 | 0,0005 | 0,002 | Remelting cathodes |
| M0 | — | 99,93 | 0,0005 | 0,004 | 0,002 | 0,003 | 0,001 | 0,002 | 0,001 | 0,003 | 0,003 | 0,04 | — | — | |
| M1 | — | 99,90 | 0,001 | 0,005 | 0,002 | 0,004 | 0,002 | 0,002 | 0,002 | 0,005 | 0,004 | 0,05 | — | — | |
| M1r | — | 99,90 | 0,001 | 0,005 | 0,002 | 0,005 | 0,002 | 0,002 | 0,002 | 0,005 | 0,005 | 0,01 | 0,002-0,012 | — | Remelting cathodes and copper scrap with phosphorus deoxidation |
| M1f | — | 99,90 | 0,001 | 0,005 | 0,002 | 0,005 | 0,002 | 0,002 | 0,002 | 0,005 | 0,005 | — | 0,012-0,04 | — | |
| M2r | — | 99,70 | 0,002 | 0,05 | 0,2 | — | 0,05 | 0,005 | 0,01 | 0,01 | 0,01 | 0,01 | 0,005-0,06 | — | |
| M3r | — | 99,50 | 0,003 | 0,05 | 0,2 | — | 0,05 | 0,05 | 0,05 | 0,03 | 0,01 | 0,01 | 0,005-0,06 | — | |
| M2 | — | 99,70 | 0,002 | 0,05 | 0,2 | — | 0,05 | 0,005 | 0,01 | 0,01 | 0,01 | 0,07 | — | — | Fire refining and smelting of copper waste and scrap |
| M3 | — | 99,50 | 0,003 | 0,05 | 0,2 | — | 0,05 | 0,05 | 0,01 | 0,05 | 0,01 | 0,08 | — | — | |
| Notes 1 In copper grades M00b and M00, the mass fraction of selenium should not exceed 0.0005%, tellurium - 0.0005%. 2 By agreement of the parties, in accordance with the contract, it is allowed to manufacture M0b grade copper with a mass fraction of oxygen not exceeding 0.002%. 3 The designation of copper grades M1 and M1p, intended for the electrical industry and subject to testing for electrical conductivity, additionally includes the letter E. 4 By agreement of the parties, in accordance with the contract, it is allowed to manufacture copper grades M00 and M0 with a mass fraction of oxygen of 0.035% and 0.045%, respectively. 5 The sign “-” means that this element is not standardized. 6 According to the contract between the manufacturer and the consumer, in products made of copper grade M1 intended for the electrical industry, the mass fraction of phosphorus should not exceed 0.002%. | |||||||||||||||
Table 2 (Changed edition, Amendment No. 1).
Use of copper in medicine
The use of copper in the medical industry can be found quite often. According to the norms of traditional medicine, copper is an extremely important element of human life. In our body, copper is present in a volume of 2 * 10-4% of a person’s total weight. Every day we consume approximately 60 mg of copper with food, but only 2 mg is absorbed, but this is the amount that is the daily norm for an adult. Copper is extremely important in the process of hemoglobin biosynthesis, as well as in maintaining sugar, cholesterol and uric acid levels. In order for the cardiovascular system, brain, and digestive tract to function as expected, copper is needed. With a chronic lack of copper in the human body, the following diseases develop:
- anemia;
- osteoporosis;
- glaucoma;
- psoriasis;
- the heart muscle atrophies;
- a person gets tired quickly and loses weight;
- Cholesterol accumulates in the body.
The richest foods containing copper are:
- Champignon;
- potato;
- Cod liver;
- whole grain;
- oysters and cuttlefish.
Pure copper
Grade M0 contains 99.95% Cu and no more than 0.05% impurities. According to special technical conditions, several grades of vacuum copper and especially oxygen-free pure copper are produced, which is used in the electric vacuum industry. Strips, tapes, rods, and pipes are produced from oxygen-free copper of series A and B. Tapes and rods are made from vacuum pure copper. Rods are produced from pure copper, which is deoxidized with manganese. All these semi-finished products are used in the electrovacuum industry. Oxygen-free pure copper is characterized by a low (-100°C) recrystallization temperature.
Appendix A (reference)
Conformity of grades according to GOST 859-2014, BS EN 1412:1996*, ISO 1190-1:1982, BS EN 1978:1998* **
________________
* Changed edition, Rev. N 1.
** Access to international and foreign documents mentioned in the text can be obtained by contacting the User Support Service. — Note from the database manufacturer.
Table A.1 - Conformity of grades according to GOST 859-2014, BS EN 1412:1996 [1] and ISO 1190-1:1982 [2], BS EN 1978:1998 [3]
| Copper grade | ||
| GOST 859- | BS EN 1412:1996 and ISO 1190-1:1982, BS EN 1978:1998 | |
| Designation | European number | |
| M00k | Cu-SATH-1 | CR001A |
| M1k | Cu-CATH-2 | CR002A |
| M00 | Cu-ETP1 | CW003A |
| M0, M1 | Cu-ETP | CW004A |
| M00b | Cu OFE1 | CW009A |
| M0b | CuOF1 | CW007A |
| M1r | Cu-DLP | CW023A |
| M1f | Cu-DHP | CW024A |
Table A.1 (Changed edition, Amendment No. 1).
Basic properties of copper
Physical properties
In air, copper acquires a bright yellowish-red hue due to the formation of an oxide film. Thin plates have a greenish-blue color when examined through them. In its pure form, copper is quite soft, malleable and easily rolled and drawn. Impurities can increase its hardness.
This is interesting: Classification of metal threads
The high electrical conductivity of copper can be called the main property that determines its predominant use. Copper also has very high thermal conductivity. Impurities such as iron, phosphorus, tin, antimony and arsenic affect the basic properties and reduce electrical and thermal conductivity. According to these indicators, copper is second only to silver.
Copper has high densities, melting points and boiling points. An important property is also good resistance to corrosion. For example, at high humidity, iron oxidizes much faster.
Copper lends itself well to processing: it is rolled into copper sheets and copper rods, and drawn into copper wire with a thickness brought to thousandths of a millimeter. This metal is diamagnetic, that is, it is magnetized against the direction of the external magnetic field.
Chemical properties
Copper is a relatively low-active metal. Under normal conditions in dry air, its oxidation does not occur. It reacts easily with halogens, selenium and sulfur. Acids without oxidizing properties have no effect on copper. There are no chemical reactions with hydrogen, carbon and nitrogen. In humid air, oxidation occurs to form copper (II) carbonate, the top layer of platinum. Copper is amphoteric, meaning it forms cations and anions in the earth's crust. Depending on the conditions, copper compounds exhibit acidic or basic properties.
Features of popular copper alloys
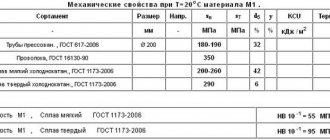
Alloy M1 is manufactured in accordance with GOST 859-2014, is a highly plastic and well-processed metal, and has the highest copper content (99.9%). Additional elements include zinc, nickel, phosphorus, iron, arsenic, oxygen, tin, bismuth (total no more than 0.1%). The electrical resistivity is 0.018 μOhm. The alloy can be of two types - hard (M1t) and soft (M1m), they differ in strength and fluidity. Rolled metal is in demand in the automotive and aircraft industries, in the creation of current conductors, cryogenic equipment, wire and rods.
Alloy M2 has a lower copper ratio in the composition (99.7%). The remaining 0.3% comes from nickel, iron, antimony, oxygen, tin, lead, sulfur, and arsenic. This grade is ductile and does not rust, is excellent in pressure processing and is used for the manufacture of copper-based alloys and refrigeration parts.
Alloy M3 is technical copper; it contains the smallest percentage of metal among those presented (99.5%). The same elements as in M2 are used as alloying components, only in a larger proportion (up to 0.5%), which makes this alloy the most affordable. Optimally suitable for metal products that are sold by rolling methods, as well as cast alloys.
Stamps
Official publication
INTERSTATE COUNCIL FOR STANDARDIZATION, METROLOGY AND CERTIFICATION
Minsk
Preface
1 DEVELOPED by the Interstate Technical Committee for Standardization MTK 503 “Copper”
INTRODUCED by Gosstandart of Russia
2 ADOPTED by the Interstate Council for Standardization, Metrology and Certification (Protocol No. 19 of May 24, 2001)
The following voted for adoption:
| State name | Name of the national standardization body |
| The Republic of Azerbaijan | Azgosstandart |
| Republic of Armenia | Armgosstandard |
| Republic of Belarus | State Standard of the Republic of Belarus |
| The Republic of Kazakhstan | Gosstandart of the Republic of Kazakhstan |
| Republic of Kyrgyzstan | Kyrgyzstandard |
| The Republic of Moldova | Moldovastandard |
| Russian Federation | Gosstandart of Russia |
| The Republic of Tajikistan | T ajikstandard |
| Turkmenistan | Main State Service "Turkmenstandartlary" |
| The Republic of Uzbekistan | Uzgosstandart |
| Ukraine | State Standard of Ukraine |
3 By Decree of the State Committee of the Russian Federation for Standardization and Metrology dated July 30, 2001 No. 301-st, the interstate standard GOST 859-2001 was put into effect directly as the state standard of the Russian Federation on March 1, 2002.
4 INSTEAD GOST 859-78
5th EDITION (February 2003) with Amendment (NUS 1-2002)
This standard cannot be fully or partially reproduced, replicated and distributed as an official publication on the territory of the Russian Federation without the permission of the State Standard of Russia
© IPC Publishing House of Standards, 2001 © IPC Publishing House of Standards, 2003 © STANDARTINFORM, 2008 Reissue (as of May 2008)
Content
1 area of use………………………………………………. 1
2 Normative references…………………………………………………………… 1
3 Technical requirements……………………………………………. 2
INTERSTATE STANDARD
COPPER
Stamps
Copper. Grades
Date of introduction 2002—03—01
1 area of use
This standard applies to copper manufactured in the form of cathodes, as well as cast and wrought semi-finished products.
The standard is suitable for certification purposes.
2 Normative references
tsam
tsam
This standard uses references to the following standards:
GOST 9717.1-82 Copper. Method of spectral analysis using metal standard images - with photoelectric recording of the spectrum
GOST 9717.2—82 Copper. Method of spectral analysis using metal standard images - with photographic registration of the spectrum GOST 9717.3-82 Copper. Method of spectral analysis for oxide standard samples GOST 13938.1-78 Copper. Methods for determining copper GOST 13938.2-78 Copper. Methods for determining sulfur GOST 13938.3-78 Copper. Method for determining phosphorus GOST 13938.4-78 Copper. Methods for determining iron GOST 13938.5-78 Copper. Methods for determining zinc GOST 13938.6-78 Copper. Methods for determining nickel GOST 13938.7-78 Copper. Methods for determining lead GOST 13938.8-78 Copper. Methods for determining tin GOST 13938.9-78 Copper. Methods for determining silver GOST 13938.10-78 Copper. Methods for determining antimony GOST 13938.11-78 Copper. Method for determination of arsenic GOST 13938.12-78 Copper. Methods for determining bismuth GOST 13938.13-93 Copper. Methods for determining oxygen GOST 13938.15-88 Copper. Methods for determination of chromium and cadmium GOST 27981.0-88 High purity copper. General requirements for methods of analysis GOST 27981.1-88 High purity copper. Methods of atomic spectral analysis GOST 27981.2-88 High purity copper. Method of chemical-atomic emission analysis GOST 27981.3-88 High-purity copper. Method of emission spectral analysis with photoelectric recording of the spectrum
GOST 27981.4—88 High purity copper. Methods of atomic absorption analysis GOST 27981.5-88 High purity copper. Photometric methods of analysis GOST 27981.6-88 High purity copper. Polarographic methods of analysis ST SEV 543-77 Numbers. Recording and rounding rules
Official publication
Biological value for humans
Copper belongs to the category of vital elements, and the body of an adult contains about 100 grams of this metal. A reassessment of the toxicity of this substance was carried out in 2003 by the World Health Organization. Studies have found that copper is not a cause of diseases of the digestive tract, and does not provoke the development of Wilson-Konovalov disease (hepatocerebral dystrophy affecting the liver and brain), as previously thought. Scientists have concluded that a lack of copper is more harmful to human health than its excess.
The bactericidal properties of copper have been known for a long time, and recent studies in this area have confirmed the effectiveness of the metal in the prevention of swine flu and infection by Staphylococcus aureus. In experiments, it was found that 99% of pathogenic bacteria die on a copper surface within 2 hours. Therefore, copper and its alloys are widely used for water disinfection. In Europe, door handles, locks, hinges and railings are made from this metal, which are installed in medical institutions and public places.
Methods for obtaining copper
In nature, copper exists in compounds and in the form of nuggets. The compounds are represented by oxides, bicarbonates, sulfur and carbon dioxide complexes, as well as sulfide ores. The most common ores are copper pyrite and copper luster. The copper content in them is 1-2%. 90% of primary copper is mined using the pyrometallurgical method and 10% using the hydrometallurgical method.
1. The pyrometallurgical method includes the following processes: enrichment and roasting, smelting for matte, purging in a converter, electrolytic refining. Copper ores are enriched by flotation and oxidative roasting. The essence of the flotation method is as follows: copper particles suspended in an aqueous medium adhere to the surface of air bubbles and rise to the surface. The method allows you to obtain copper powder concentrate, which contains 10-35% copper.
Copper ores and concentrates with a significant sulfur content are subject to oxidative roasting. When heated in the presence of oxygen, sulfides are oxidized, and the amount of sulfur is reduced by almost half. Poor concentrates containing 8-25% copper are roasted. Rich concentrates containing 25-35% copper are melted without resorting to roasting.
The next stage of the pyrometallurgical method for producing copper is smelting for matte. If lump copper ore with a large amount of sulfur is used as a raw material, then smelting is carried out in shaft furnaces. And for powdered flotation concentrate, reverberatory furnaces are used. Melting occurs at a temperature of 1450 °C.
In horizontal converters with side blowing, the copper matte is blown with compressed air in order for the oxidation of sulfides and ferrum to occur. Next, the resulting oxides are converted into slag, and sulfur into oxide. The converter produces blister copper, which contains 98.4-99.4% copper, iron, sulfur, as well as small amounts of nickel, tin, silver and gold.
This is interesting: Description of steel 40X
Blister copper is subject to fire and then electrolytic refining. Impurities are removed with gases and converted into slag. As a result of fire refining, copper is formed with a purity of up to 99.5%. And after electrolytic refining, the purity is 99.95%.
2. The hydrometallurgical method involves leaching copper with a weak solution of sulfuric acid, and then separating copper metal directly from the solution. This method is used for processing low-grade ores and does not allow for the associated extraction of precious metals along with copper.
Copper alloys, their properties, characteristics, grades
The production of copper alloys makes it possible to improve the properties of copper without losing the main advantages of this metal, as well as to obtain additional useful properties.
Copper alloys include: bronze, brass and copper-nickel alloys.
Brass
This is an alloy of copper and zinc. In addition to zinc, it also contains other alloying additives, including tin.
Brasses are corrosion-resistant alloys. They have anti-friction properties to resist vibrations. They have high fluidity rates, which gives products made from them a high degree of resistance to heavy loads. In brass castings, segregation areas are practically not formed, so the products have a uniform structure and density.
Brasses are marked with a set of alphanumeric codes, where the first letter is always L, meaning brass itself. Next comes a digital indicator of the percentage of copper in brass. The remaining letters and numbers indicate the content of alloying elements as a percentage. Brasses use the same letter designations for alloying elements as bronzes.
An example of double brass marking: L85. It stands for “brass with a copper content of up to 85%, the rest is zinc.”
An example of multi-component brass marking: LMtsA57-3-1. It stands for “brass with a copper content of up to 57%, manganese - up to 3%, aluminum - up to 1%, the rest is zinc.”
Bronze
An alloy of copper and tin. However, with the development of technology, bronzes also appeared, in which, instead of tin, aluminum, silicon, beryllium and lead were introduced into the alloy.
Bronze is harder than copper. They have higher strength ratings. They are better suited to metal forming, especially forging.
Marking of bronzes is carried out using alphanumeric codes, where the first letters are Br, meaning bronze itself. Additional letters indicate alloying elements, and numbers after the letters indicate the percentage of such elements in the alloy.
Letter designations of bronze alloying elements:
- A – aluminum,
- B – beryllium,
- F – iron,
- K – silicon,
- Mts – manganese,
- N – nickel,
- O - tin,
- C – lead,
- C – zinc,
- F – phosphorus.
An example of marking tin bronze: BrO10S12N3. It stands for “tin bronze with a tin content of up to 10%, lead – up to 12%, nickel – up to 3%.”
An example of decoding aluminum bronze: BrAZh9-4. It stands for “aluminum bronze with an aluminum content of up to 9% and iron up to 4%.”
Copper-nickel alloys
- Cupronickel is an alloy of copper and nickel. Iron and manganese may be present in the alloy as additives. Special cases of technical alloys based on copper and nickel:
- Nickel silver – additionally contains zinc,
- Constantan – additionally contains manganese.
Cupronickel has high corrosion resistance. It lends itself well to any type of mechanical processing. Non-magnetic. It has a pleasant silver color.
Due to its properties, cupronickel is, first of all, a decorative and applied material. Jewelry and souvenirs are made from it. For decorative purposes it is an excellent substitute for silver.
There are 2 brands of cupronickel available:
- MNZHMts – an alloy of copper with nickel, iron and manganese;
- MH19 is an alloy of copper and nickel.