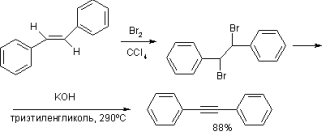
Properties and production of acetylene
Under atmospheric pressure and normal temperature, acetylene is a colorless gas. If the temperature drops to -85 degrees or lower, then this compound transforms into another state - solid. In this case, crystals are formed. It should be noted that in liquid and solid states, acetylene can easily explode under the influence of friction or impact (hydraulic or mechanical). It is this property that largely determines its scope of application. Acetylene combustion reactions occur in the presence of oxygen. As a result of this process, a flame appears that is characterized by the highest temperatures (3150 degrees) compared to other types of fuel.
The main method for producing acetylene is a reaction in which calcium carbide and water react. This process occurs at temperatures of about 2000 degrees and is endothermic.
There is such a thing as acetylene yield. This is the amount that is released as a result of the decomposition of 1 kg of calcium carbide. GOST 1460-56 establishes specific values for this value, which is directly dependent on the degree of granulation of the starting substance. Thus, a consequence of the relatively small particle size of calcium carbide is a decrease in the yield of acetylene.
This pattern is a consequence of the presence of foreign impurities, such as calcium oxide, in small carbide particles.
There are other, less bulky, expensive and energy-intensive methods for producing acetylene. For example, the reaction of thermal-oxidative pyrolysis of methane from natural gas; decomposition of oil, kerosene and other types of fuel by electropyrolysis.
ACETYLENE
ACETYLENE (ethyne) is a hydrocarbon of composition C2H2 containing a triple carbon-carbon bond. The name of this compound has been familiar not only to chemists for more than a hundred years. Since the end of the 19th century, when a cheap method for producing acetylene from calcium carbide (CaC2 + 2H2O ® C2H2 + Ca(OH)2) was developed, this gas began to be used for lighting. In a flame at high temperatures, acetylene, containing 92.3% carbon (this is a kind of chemical record), decomposes to form solid carbon particles, which can contain from several to millions of carbon atoms. Heating strongly in the inner cone of the flame, these particles cause the flame to glow brightly - from yellow to white, depending on the temperature (the hotter the flame, the closer its color is to white). Acetylene torches produced 15 times more light than conventional gas lamps that illuminated the streets. Gradually they were replaced by electric lighting, but for a long time they were used in small lamps on bicycles, motorcycles, and in horse-drawn carriages.
Also on topic:
CHEMICAL ELEMENTS
Acetylene was first produced in 1836 by Edmund Davy, cousin of the famous Humphry Davy. He reacted water on potassium carbide: K2C2 + H2O ® C2H2 + 2KOH and obtained a new gas, which he called hydrogen bicarbonate. It was mainly of interest to chemists from the point of view of the theory of the structure of organic compounds. One of the creators of the so-called theory of radicals, Justus Liebig, called a group of atoms (i.e. radical) C2H3 acetyl. In Latin, acetum means vinegar; the acetic acid molecule (C2H3O+O+H, as its formula was written then) was considered as an acetyl derivative. When the French chemist Marcelin Berthelot in 1855 managed to obtain “hydrogen bicarbonate” by several methods at once, he named it acetylene. Berthelot considered acetylene to be a derivative of acetyl from which one hydrogen atom was removed: C2H3 – H ® C2H2. (Now the CH3CO group is called acetyl; it is part of the salts of acetic acid - acetates, as well as acetone CH3CO-CH3, acetaldehyde CH3CO-H, acetyl chloride CH3CO-Cl and many other compounds.)
First, Berthelot obtained acetylene by passing vapors of ethylene, methyl and ethyl alcohol through a red-hot tube. In 1862, he managed to synthesize acetylene from elements by passing hydrogen through a voltaic arc flame between two carbon electrodes, and in 1867 he made a very important discovery for chemical theory: he showed that from three acetylene molecules one can obtain a benzene molecule: 3C2H2 ® C6H6.
Also on topic:
CARBON
All the mentioned synthesis methods had only theoretical significance, and acetylene was a rare and expensive gas until a cheap method was developed for producing calcium carbide by calcining a mixture of coal and quicklime: CaO + 3C ® CaC2 + CO. For a long time, acetylene for technical needs (for example, at construction sites) was obtained by “quenching” carbide with water. Acetylene obtained from technical calcium carbide has an unpleasant odor due to impurities of ammonia, hydrogen sulfide, phosphine PH3, arsine AsH3. Methods for producing acetylene from natural gas - methane - are now widely used: electrocracking 2CH4 ® C2H2 + 3H2 (a stream of methane is passed between the electrodes at a temperature of 1600°C and quickly cooled to prevent the decomposition of acetylene); thermal oxidative cracking (incomplete oxidation) 6CH4 + 4O2 ® C2H2 + 8H2 + 3CO + CO2 + 3H2O (the heat of partial combustion of acetylene is used in the reaction).
Pure acetylene, when cooled, liquefies at –83.8° C, and with a further decrease in temperature it quickly solidifies. It is moderately soluble in water (1150 ml in 1 liter of water at 15 ° C and atmospheric pressure) and well in organic solvents, especially in acetone (25 liters in 1 liter of acetone under the same conditions and 300 liters under a pressure of 12 atm). Thermodynamically, acetylene is unstable; it explodes when heated to 500 ° C, and at ordinary temperatures - when the pressure increases to 2 atm. Therefore, it is stored in cylinders filled with porous inert material, which is impregnated with acetone.
Also on topic:
EXPLOSIVES
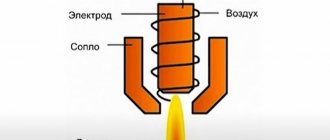
Acetylene is used for so-called autogenous welding and cutting of metals. To do this, you need two cylinders with gases - with oxygen (it is colored blue) and with acetylene (white). Gases from the cylinders enter a special burner. Back in 1895, it was discovered that burning acetylene in oxygen produced a very hot flame; its maximum temperature (3150° C) is achieved with an acetylene content of 45% by volume. In such a flame, even thick pieces of steel melt very quickly.
The chemistry of acetylene was first studied in detail in the works of Academician A.E. Favorsky (1860–1945). It turned out that acetylene can serve as a starting product for the synthesis of many more complex organic compounds. This area of application of acetylene is currently the most extensive. Acetylene is a reactive compound that undergoes numerous reactions. In 1881, M.G. Kucherov discovered the reaction of adding water to acetylene in the presence of a catalyst - mercury salts, resulting in the formation of acetaldehyde: C2H2 + H2O ® CH3CHO. From acetaldehyde, acetic acid, acetone, and alcohol are further obtained.
In 1949, the German chemist V.Yu.Reppe discovered the important reaction of carbonylation (addition of CO) of acetylene in the presence of a nickel catalyst: C2H2 + CO + H2O ® CH2=CH–COOH. The unsaturated acrylic acid formed in this reaction is used to produce a variety of polymers - acrylates (this also includes organic glass - polymethyl methacrylate). And the addition of hydrocyanic acid to acetylene gives another important product - acrylic acid nitrile (acrylonitrile): C2H2 + HCN ® CH2=CH–CN. Its polymerization produces very important polyacrylonitrile polymers, from which artificial fibers, plastics, and rubbers are made.
Halogens and hydrohalogens (the latter in the presence of catalysts) easily add to acetylene to form first substituted ethylene, then ethane, for example: HCєCH + Cl2 ® ClCH=CHCl, ClCH=CHCl + Cl2 ® CHCl2–CHCl2; HCєCH + HCl ® CH2=CHCl, CH2=CHCl + HCl ® CH3–CHCl2 (the last two reactions follow Markovnikov’s rule). The resulting chlorine derivatives are widely used as intermediates for further syntheses, and also as solvents (for example, in dry cleaning).
Acetylene is a weak acid; in the presence of strong bases, ionization of this bond is possible to form the acetylenide ion НєС–С–. Ionization of the second C–H bond is also possible, therefore, when acetylene is passed into ammonia solutions of silver and copper(I) salts, white silver acetylide C2Ag2 and red-brown copper acetylide C2Cu2 are formed. Both compounds are insoluble and precipitate; in dry form they are explosives. Calcium carbide CaC2 can also be considered as an acetylenide. These salt-like compounds have an ionic crystal lattice, the nodes of which contain metal cations and C22– anions.
In an acidic environment in the presence of Cu+ ions, acetylene dimerizes to form vinyl acetylene HCєC–CH=CH2.
When it polymerizes, products are formed that are used in the production of paints and varnishes - vinyl and didivinylacetylene varnishes.
The examples given do not exhaust the rich chemistry of acetylene, from which hundreds of different compounds can be obtained. No wonder its annual production exceeds 5 million tons. Of these, approximately 70% are used for industrial organic synthesis, and 30% are used for welding and cutting metals.
Ilya Leenson
Storage and transportation
All storage and transportation methods involve the use of cylinders. They are filled with a special mass of porous consistency. It is impregnated with acetone, which dissolves acetylene well. The use of this method can significantly increase the filling capacity of an acetylene cylinder and, importantly, reduces its explosion hazard.
Prolonged contact of acetylene with metals such as copper and silver can lead to an increase in its explosion hazard. Therefore, the use of materials that may contain these metals, for example in valves, is prohibited.
As a rule, cylinders must have special valves designed specifically for acetylene storage.
Full use of the entire container capacity can be achieved by storing empty containers so that the acetone is distributed throughout the entire volume of the container. And this is only possible in a horizontal position. Filling the cylinder must occur very slowly, which is important to comply with the conditions of the chemical reaction of dissolving acetylene in acetone, and in particular its speed.
Welding technology and modes
Acetylene-oxygen mixtures are used to join parts made of carbon and low-alloy steels. For example, this method is widely used to create permanent pipeline connections. For example, pipes with a diameter of 159 mm with a wall thickness of no more than 8 mm. But there are also some restrictions; joining steel grades 12×2M1, 12×2MFSR using this method is unacceptable.
Selecting mode parameters
To prepare the mixture necessary for combining metals, use the formula 1/1,2. When processing workpieces made of alloy steels, the welder must monitor the state of the flame. In particular, an excess of acetylene should not be allowed.
The consumption of the mixture with the oxygen/acetylene formula is 100-130 dm 3 /hour per 1 mm of thickness. The flame power is regulated using a burner, which is selected depending on the material used, its characteristics, thickness, etc.
To perform welding with acetylene, welding wire is used. Its grade must correspond to the steel grade of the parts being welded. The diameter of the wire is determined depending on the thickness of the metal being welded.
For the convenience of technologists and welders themselves, there are many tables on the basis of which you can quite easily select a welding mode. To do this you need to know the following parameters:
- wall thickness of welded workpieces;
- type of welding - left, right;
Read also: Fireclay clay crucible
Based on this, you can determine the diameter of the filler wire and select the acetylene consumption. For example, the thickness is 5-6 mm, tip No. 4 will be used to perform the work. That is, based on the tabular data, the wire diameter will be 3.5 mm for left welding, 3.5 mm for right welding. Acetylene consumption in this case will be for left welding method 60-780 dm 3 / hour, with the right 650-750 dm 3 / hour.
Welding is performed in small sections of 10-15 mm. The work is performed in the following sequence. At the first stage, the edges are melted. After this, the root suture is applied. Once the root formation is complete, welding can continue. If the thickness of the workpieces is 4 mm, then welding can be performed in one layer. If the thickness exceeds the specified one, then a second one must be applied. It is laid only after the root of the seam has been completed along the entire specified length.
To improve the quality of welding, preheating is allowed. That is, the future welded joint is heated using a torch. If this method is adopted as a basis, then warming up must be done again after each stop.
Seams can be made with gas in any spatial position. For example, when making a vertical seam there are some peculiarities. So, the vertical seam should be made from bottom to top.
When performing welding work, breaks in work are unacceptable, at least until the entire seam is cut. When stopping operation, the burner must be withdrawn slowly, otherwise seam defects - cavities and pores - may occur. An interesting feature exists when welding pipelines; a draft is not allowed in it and therefore the ends of the pipes must be plugged.
Benefits of dissolved acetylene
The main advantage of dissolved acetylene over that produced using portable calcium carbide generators is that when using cylinders, welder labor increases by approximately 20%, and acetylene losses are reduced by 25%. Also worth noting is the increased efficiency and maneuverability of the welding station, as well as safety. Unlike gas obtained from calcium carbide, dissolved acetylene contains significantly less foreign substances, that is, impurities, which makes it possible to use it in particularly critical welding work.
Molecular formula of acetylene
Ethine is the simplest member of its homologous series; its composition and structure are reflected by the formulas:
- C2H2 is the molecular notation for the composition of ethyne, which gives the idea that the substance is formed by two carbon atoms and the same number of hydrogen atoms. Using this formula, you can calculate the molecular and molar mass of a compound. Mr (C2H2) = 26 a. e.m., M (C2H2) = 26.04 g/mol.
- H:S:::S:H is the electron dot formula of acetylene. Such images, called “Lewis structures,” reflect the electronic structure of the molecule. When writing, you must follow the rules: the hydrogen atom, when forming a chemical bond, tends to have the configuration of the helium valence shell, other elements - an octet of outer electrons. Each colon represents a shared or lone pair of electrons in the outer energy level.
- H—C≡C—H is the structural formula of acetylene, reflecting the order and multiplicity of bonds between atoms. One dash replaces one pair of electrons.
Acetylene: application in construction and industry
Autogenous and welding works accompany almost all stages of construction. It is in these types of work that acetylene is used. In a special device called a burner, gases are mixed and the combustion reaction itself occurs. The highest temperature of this reaction is achieved when the acetylene content is 45% of the total volume of the cylinder.
Cylinders with this gas are marked as follows: they are painted white and the inscription “Acetylene” is written in large red letters.
Construction work is carried out mainly outdoors. The use of acetylene and its homologues under these conditions should not be exposed to direct sunlight. Short breaks should be accompanied by closing the valves on the burner, and long breaks should be accompanied by closing the valves on the cylinders themselves.
Read also: Attachment to the grinder chain saw
Acetylene is in great demand in the chemical industry. Its application lies in the use of this substance in the process of obtaining organic synthesis products. These are synthetic rubber, plastics, solvents, acetic acid, etc.
Acetylene, being a universal fuel, is often used in processes involving gas-flame processing. It is important that the use of acetylene in industry is possible only if safety measures are observed, since it is an explosive gas.
Alkyne oxidation
Oxidation reactions in organic chemistry are accompanied by an increase in the number of oxygen atoms (or the number of bonds with oxygen atoms) in the molecule and/or a decrease in the number of hydrogen atoms (or the number of bonds with hydrogen atoms).
2.1. Combustion of alkynes
Alkynes, like other hydrocarbons, burn to form carbon dioxide and water.
The general equation for the combustion of alkynes is:
2.2. Oxidation of alkynes with strong oxidizing agents
Alkynes react with strong oxidizing agents (permanganates or chromium (VI) compounds). In this case, oxidation of the C≡C triple bond and C-H bonds at carbon atoms at the triple bond . In this case, bonds with oxygen are formed.
When three bonds are oxidized at a carbon atom in an acidic environment, a carboxyl group COOH is formed, and four bonds form carbon dioxide CO2. In a neutral environment - a salt of a carboxylic acid and a carbonate (bicarbonate), respectively.
Correspondence table between the oxidized fragment of the molecule and the product:
When butine-2 is oxidized with potassium permanganate in sulfuric acid, two CH3–C ≡ fragments undergo oxidation, therefore acetic acid is formed:
When 3-methylpentine-1 is oxidized with potassium permanganate in sulfuric acid, the R–C and H–C fragments undergo oxidation, therefore carboxylic acid and carbon dioxide are formed:
When alkynes are oxidized by strong oxidizing agents in a neutral environment, carbon-containing products of the hard oxidation reaction (acid, carbon dioxide) can react with the alkali formed in the solution in a ratio determined by the electronic balance with the formation of the corresponding salts.
Similar organic products are formed when alkynes react with chromates or dichromates.
The oxidation of acetylene proceeds a little differently; the C–C σ bond is not broken, so oxalic acid is formed in an acidic environment:
In a neutral environment, a salt of oxalic acid is formed - potassium oxalate:
Discoloration of a solution of potassium permanganate is a qualitative reaction to a triple bond.
Carbide lamps
The name “carbide lamp” is due to the use of a jet of burned acetylene as an open flame as a light source. It is, accordingly, obtained as a result of the interaction of calcium carbide with water.
Such lamps were widespread in the past. They could be seen on carriages, cars and even bicycles. In modern times, carbide lamps are used only in cases of urgent need for a powerful autonomous lamp. So, speleologists often use them. Remote lighthouses are equipped with just such lamps, because this type of lighting is much more profitable than installing power lines. The use of such lamps on long-distance vessels is quite common.
Nomenclature
Alkynes and acetylene hydrocarbons are hydrocarbons whose molecules contain at least two carbon atoms that are in a state of sp-hybridization and connected to each other by three bonds.
Alkynes form a homologous series with the general formula CnH2n-2.
The first member of the homologous series is acetylene, which has the molecular formula C2H2 and the structural formula CHCH. Due to the peculiarity of sp-hybridization, the acetylene molecule has a linear structure. The presence of two π-bonds located in two mutually perpendicular planes implies the location of the α-atoms of the substituent groups on the line of intersection of the planes in which the π-bonds are located. Therefore, the bonds of carbon atoms spent on connection with other atoms or groups are rigidly located on a line at an angle of 1800 to each other. The structure of the triple bond system in alkyne molecules is determined by their linear structure.
The structural feature of alkyne molecules suggests the existence of isomerism in the position of the triple bond. Structural isomerism, due to the structure of the carbon skeleton, begins with the fifth member of the homologous series.
1. Isomerism of the triple bond position. For example:
2. Structural isomers. For example:
The first member of the homologous series has the trivial name “acetylene”.
According to rational nomenclature, acetylene hydrocarbons are considered as acetylene derivatives. For example:
According to the IUPAC nomenclature, the names of alkynes are formed by replacing the suffix “an” with “in”. The main chain is chosen so that it contains a triple bond. The numbering of carbon atoms begins from the end of the chain to which the triple bond is closest. If there are double and triple bonds in a molecule, the double bond has a lower number. For example:
The triple bond can be terminal (terminal, such as in propyne) or "internal", such as in 4-methyl-2-pentine.
When naming, the radical -CCH is called “ethynyl”.
Acetylene: medical uses
How is the substance used in this area? General anesthesia involves the use of alkynes. Acetylene is one of the gases used in inhalation anesthesia. But its widespread use in this capacity is a thing of the past. Now there are more modern and safer methods of anesthesia.
Although it should be noted that the use of acetylene did not pose a great danger, since before the value of its concentration in the inhaled air reaches a dangerous limit, the lower threshold of flammability will be passed.
The most important condition for using this gas is compliance with safety measures. It is difficult to overestimate how dangerous acetylene is. Its use is possible only after all necessary training has been carried out with workers in the various fields in which it is used.
Under conditions of normal air humidity and temperature, acetylene is a colorless gas that is produced in stationary generators by the action of water on calcium carbide. When the temperature drops to -85 degrees Celsius, the substance turns into a solid state, and at the same time crystals form. An important property of acetylene is that it explodes upon impact or friction. This parameter largely determines the scope of use.
Characteristics
The decomposition of acetylene is an exothermic reaction. Its calorific value is 24,000 kcal/. Likewise, its synthesis usually requires high temperatures in some steps or the contribution of chemical energy in some other way.
Acetylene is an explosive gas if its content in the air is from 2 to 82%. It also explodes when compressed on its own without dissolving in another substance, so for storage it is dissolved in acetone, a liquid solvent that stabilizes it.
Welding work using acetylene
Traditionally, this gas is used when carrying out autogenous welding procedures, as well as cutting metals. The technology involves the use of two gas cylinders, one of which contains oxygen, and the other contains acetylene. The substances enter a specialized burner, and during combustion a very hot flame is formed. Its temperature can reach 3200 degrees Celsius. The most “effective” combination of gases is considered to be one in which the mixture contains 45% acetylene. Under such conditions, it is possible to quickly melt even fairly thick pieces of sheet steel.
Advantages of acetylene in flame processing of metals
The use of acetylene for gas-flame processing of metals faces strong competition from more accessible combustible gases (natural gas, propane-butane, etc.). However, the advantage of acetylene is the highest combustion temperature, which reaches 3200 ° C. That is why gas-flame processing of critical components of mechanical engineering structures is carried out only with the help of acetylene, which ensures the highest productivity and quality of the welding process.
Read also: What is rope reeving
Comparative flame characteristics when welding with different gases
| Gas | Flame temperature, °C |
| Acetylene | 3000 – 3200 |
| MAF | 2930 |
| Propane | 2600-2750 |
| Hydrogen | 2100-2500 |
| Methane | 2000-2200 |
Use in industry and everyday life
However, autogenous welding and cutting of metals is not the only area of application. Quite often, acetylene is used as a source of bright white light in self-contained lighting devices. In this case, it is obtained by the reaction of water and calcium carbide.
Such lamps were in great demand in the last century; they were used to illuminate carriages and cars. But even today, carbide devices, that is, those created using acetylene, are used in the improvement of remote lighthouses. The key advantage of carbide lamps is their efficiency and the absence of the need to connect to an electrical outlet. Accordingly, when installing them on a lighthouse, there is no need to connect a power line, that is, to pay for an expensive service. Lamps are also in demand on long-distance vessels.
Acetylene is used in industry. It is used in the preparation of various organic synthesis products. For example, it is used to create:
- acetic acid;
- synthetic rubber;
- solvents;
- some types of plastics.
It should be noted that acetylene has also found application in medicine, for example, it is sometimes used for inhalation anesthesia.
General information
Acetylene is an unsaturated hydrocarbon C 2 H 2 . It has a triple bond between carbon atoms and belongs to the class of alkynes. It is practically not found in nature on Earth, because Due to the presence of oxygen, this extremely unstable compound is obtained by synthesis. Acetylene has been found in the atmospheres of Uranus, Jupiter and Saturn.
Read also: At what height from the floor to hang sconces
Gaseous acetylene was first obtained in 1836 by Edmund Davy during the decomposition of potassium carbide with water, obtained by fusing potassium metal with coal: K 2 C 2 + 2 H 2 O = C 2 H 2 + 2 KOH.
Since the end of the 19th century, when a cheap method was developed for producing acetylene from calcium carbide (CaC 2 + 2H 2 O = C 2 H 2 + Ca(OH) 2, which in turn was obtained by calcining a mixture of coal and quicklime (CaO + 3C = CaC 2 + CO), this gas began to be used for lighting. In a flame at high temperature, acetylene, containing 92.3% carbon (this is a kind of chemical record), decomposes to form solid carbon particles, which can contain from several to millions of carbon atoms. Strongly heated in the inner cone of the flame, these particles cause a bright glow of the flame - from yellow to white, depending on the temperature (the hotter the flame, the closer to white its color). Acetylene torches gave 15 times more light than ordinary gas lamps that illuminated the streets.Gradually they were replaced by electric lighting, but for a long time they were used in small lamps on bicycles, motorcycles, and horse-drawn carriages.
Content
- 1 Receipt
- 1.1 In the laboratory
- 1.2 In industry
- 1.2.1 Preparation by pyrolysis
- 1.2.1.1 Electrocracking
- 1.2.1.2 Regenerative pyrolysis
- 1.2.1.3 Oxidative pyrolysis
- 1.2.1.4 Homogeneous pyrolysis
- 1.2.1.5 Pyrolysis in a low-temperature plasma jet
- 1.2.2 Carbide method
- 2 Physical properties
- 3 Chemical properties
- 4 History
- 5 Application
- 6 Security
- 7 Notes
- 8 Literature
- 9 Links
Read also: Is it possible to connect a difavtomat from below
Receipt
In the laboratory
In the laboratory, acetylene is produced by the action of water on calcium carbide, see video of this process (F. Wöhler, 1862),
as well as during the dehydrogenation of two methane molecules at temperatures above 1400 °C:
In industry
In industry, acetylene is produced from calcium carbide and pyrolysis of hydrocarbon raw materials - methane or propane with butane. In the latter case, acetylene is produced together with ethylene. The carbide method allows one to obtain pure acetylene, but requires high power consumption. Pyrolysis is less energy intensive, but the resulting acetylene has a low concentration in the gas stream and requires separation. Economic evaluations of both methods are numerous but controversial.
Preparation by pyrolysis
Methane is converted into acetylene and hydrogen in electric arc furnaces (temperature 2000-3000°C, voltage between electrodes 1000 V). Methane heats up to 1600°C. Electricity consumption is about 13,000 kWh per 1 ton of acetylene, which is relatively high (approximately equal to the energy consumed by the carbide method) and therefore is a disadvantage of the process. The acetylene yield is 50%.
Another name is the Wolf process. First, the furnace head is heated by burning methane at 1350-1400°C. Next, methane is passed through the heated nozzle. The residence time of methane in the reaction zone is very short and amounts to a fraction of a second. The process was implemented in industry, but economically it turned out to be not as promising as it was thought at the design stage.
Methane is mixed with oxygen. Part of the raw material is burned, and the resulting heat is used to heat the rest of the raw material to 1600°C. The acetylene yield is 30-32%. The method has the advantages of a continuous process and low energy consumption. In addition, synthesis gas is also formed with acetylene. This process (Sachse process or BASF process) has received the most widespread adoption.
It is a type of oxidative pyrolysis. Part of the raw material is burned with oxygen in the furnace firebox, the gas is heated to 2000°C. Then the remainder of the raw material, preheated to 600°C, is introduced into the middle part of the furnace. Acetylene is formed. The method is characterized by greater safety and reliability of the furnace.
Pyrolysis in a low-temperature plasma jet
The process has been developed since the 1970s, but, despite its promise, has not yet been introduced into industry. The essence of the process is heating methane with ionized gas. The advantage of the method is its relatively low energy consumption (5000-7000 kWh) and high yields of acetylene (87% in argon plasma and 73% in hydrogen plasma).
Carbide method
This method has been known since the 19th century, but has not lost its importance to this day. First, calcium carbide is obtained by fusing calcium oxide and coke in electric furnaces at 2500-3000°C:
Lime is obtained from calcium carbonate:
Next, calcium carbide is treated with water:
The resulting acetylene has a high degree of purity of 99.9%. The main disadvantage of the process is the high energy consumption: 10,000-11,000 kWh per 1 ton of acetylene.
References
- CAS number
- Matheson Gas Handbook. "Lower and Upper Explosion Limits for Flammable Gases and Vapors (LEL/LEL)" (PDF) (English). Matheson Gas Products. paragraph 443. Consultado el October 2, 2016.
- Henry Enfield Roscoe and Karl Schorlemmer. A Treatise on Chemistry, D. Appleton and Co., 1833, p. 614, in which reference is made to the Reports of the British Association,
1836, p. 62. - “Because of the brightness with which the new gas burns on contact with the atmosphere, it is, in the opinion of the author, admirably adapted for artificial illumination if it can be obtained at a low cost.” William Joseph Dibdin. "Acetylene", Public Lighting by Gas and Electricity,
Ch. XXIX, p. 489. - American Council of Learned Societies. Dictionary of Scientific Biography,
Charles Scribner's Sons, New York, 1981, Vol. 2, p.67. - Popular illustrated encyclopedic dictionary Salvat (1906 - 1914)
Bibliography
- National Institute of Occupational Safety and Health of Spain: International Chemical Safety Data Sheet for Acetylene.
| authoritative control |
|
- Datos: Q133145
- Multimedia: Acetylene
Physical properties
Under normal conditions, it is a colorless gas, lighter than air. Pure 100% acetylene is odorless, but technical acetylene contains impurities that give it a pungent odor. Slightly soluble in water, soluble in acetone. Boiling point −83.6 °C. Triple point −80.55 °C at a pressure of 961.5 mmHg. Art., critical point 35.18 °C at a pressure of 61.1 atm.
Acetylene requires great care when handling. May explode on impact, when heated to 500 °C, or when compressed to 1.4 atm at room temperature. A stream of acetylene released into the open air can ignite from the slightest spark, including from a discharge of static electricity from a finger. To store acetylene, special cylinders filled with porous material impregnated with acetone are used.
Acetylene has been discovered on Uranus and Neptune.
Addition reactions
A triple bond consists of a σ bond and two π bonds. Let's compare the characteristics of a single C–C bond, a triple bond C ≡ C, and a C–H bond:
Thus, the C≡C triple bond is shorter than the C–C single bond, therefore the π-electrons of the triple bond are held more tightly by the nuclei of carbon atoms and have less polarizability and mobility. Triple bond addition reactions
Alkynes are characterized by addition reactions at the triple bond C ≡ C with breaking of π bonds.
1.1. Hydrogenation
The hydrogenation of alkynes occurs in the presence of catalysts (Ni, Pt) with the formation of alkenes, and then immediately alkanes.
When using a less active catalyst (Pd, CaCO3, Pb(CH3COO)2), hydrogenation stops at the stage of formation of alkenes.
1.2. Halogenation of alkynes
The addition of halogens to alkynes occurs even at room temperature in solution (solvents - water, CCl4).
Alkynes react similarly with chlorine, but chlorine water does not become discolored, because chlorine water is already colorless)
Reactions occur in the presence of polar solvents according to an ionic (electrophilic) mechanism.
1.3. Hydrohalogenation of alkynes
Alkynes add hydrogen halides. The reaction proceeds by the mechanism of electrophilic addition with the formation of a halogenated alkene or dihaloalkane.
When hydrogen halides and other polar molecules add to symmetrical alkynes, as a rule, one reaction product is formed, where both halogens are located at the same C atom.
When polar molecules add to unsymmetrical alkynes, a mixture of isomers is formed. In this case, Markovnikov's rule is satisfied.
1.4. Alkyne hydration
Hydration (addition of water) of alkynes occurs in the presence of an acid and a catalyst (mercury salt II).
First, an unstable alkene alcohol is formed, which then isomerizes to an aldehyde or ketone.
The hydration of alkynes occurs via an ionic (electrophilic) mechanism.
For unsymmetrical alkenes, the addition of water predominantly follows the Markovnikov rule.
1.5. Dimerization, trimerization and polymerization
The addition of one acetylene molecule to another ( dimerization ) occurs under the influence of an ammonia solution of copper (I) chloride. This produces vinyl acetylene:
Trimerization of acetylene (the addition of three molecules to each other) occurs under the influence of temperature, pressure and in the presence of activated carbon with the formation of benzene (Zelinsky reaction):
Alkynes also undergo polymerization - the process of repeatedly combining molecules of a low molecular weight substance (monomer) with each other to form a high molecular weight substance (polymer).
nM → Mn ( M is a monomer molecule)
… –CH=CH–CH=CH–CH=CH–…
Chemical properties
Acetylene (ethyne) is characterized by addition reactions:
HC≡CH + Cl2 -> СlСН=СНCl
Acetylene with water, in the presence of mercury salts and other catalysts, forms acetaldehyde (Kucherov reaction). Due to the presence of a triple bond, the molecule is high-energy and has a high specific heat of combustion - 14,000 kcal/m³ (50.4 MJ/Kg). When burned in oxygen, the flame temperature reaches 3150 °C. Acetylene can polymerize into benzene and other organic compounds (polyacetylene, vinyl acetylene). Polymerization into benzene requires graphite and a temperature of 400 °C.
In addition, the hydrogen atoms of acetylene are relatively easily eliminated in the form of protons, that is, it exhibits acidic properties. Thus, acetylene displaces methane from an ethereal solution of methylmagnesium bromide (a solution containing acetylene ion is formed), and forms insoluble explosive precipitates with salts of silver and monovalent copper.
The main chemical reactions of acetylene (addition reactions, summary table 1.):
Read also: How to disassemble an electric motor armature
The main chemical reactions of acetylene (addition reactions, dimerization, polymerization, cyclomerization, summary table 2.):
Acetylene decolorizes bromine water and potassium permanganate solution.
Reacts with ammonia solutions of Cu(I) and Ag(I) salts to form poorly soluble, explosive acetylenides - this reaction is used for the qualitative determination of acetylene and its difference from alkenes (which also decolorize bromine water and potassium permanganate solution).
Acetylene molecule models
Formulas showing the distribution of electrons served as the foundation for the creation of atomic orbital models and spatial formulas of molecules (stereochemical). At the end of the 18th century, ball-and-stick models became widespread - for example, balls of different colors and sizes, indicating carbon and hydrogen, which form acetylene. The structural formula of a molecule is presented in the form of rods, symbolizing chemical bonds and their number in each atom.
The ball-and-stick model of acetylene reproduces bond angles equal to 180°, but the internuclear distances in the molecule are reflected approximately. The voids between the balls do not create the idea of filling the space of atoms with electron density. The shortcoming is eliminated in Dreiding's models, which designate the nuclei of atoms not as balls, but as points of attachment of rods to each other. Modern three-dimensional models provide a clearer picture of atomic and molecular orbitals.
Application
Safety
Because acetylene is insoluble in water and its mixtures with oxygen can explode over a very wide range of concentrations, it cannot be collected in gasometers.
Acetylene explodes at temperatures around 500 °C or pressures above 0.2 MPa; CPV 2.3-80.7%, auto-ignition temperature 335 °C. Explosiveness is reduced when acetylene is diluted with other gases, such as nitrogen, methane or propane.
When acetylene comes into contact with copper and silver for a long time, copper and silver acetylenides are formed, which explode upon impact or increased temperature. Therefore, when storing acetylene, materials containing copper (for example, cylinder valves) are not used.
Acetylene has a weak toxic effect. For acetylene, the maximum permissible concentration limit is normalized. = MPC s.s. = 1.5 mg/m3 according to hygienic standards GN 2.1.6.1338-03 “Maximum permissible concentrations (MAC) of pollutants in the atmospheric air of populated areas.”
MPCr.z. (working area) is not established (according to GOST 5457-75 and GN 2.2.5.1314-03), since the concentration limits of flame distribution in a mixture with air are 2.5-100%.
It is stored and transported in white steel cylinders filled with an inert porous mass (for example, charcoal) (with a red lettering “A”) in the form of a solution in acetone under a pressure of 1.5-2.5 MPa.
Acetylene cylinders
Structural and molecular formula: acetylene
Gas cylinders, incl. acetylene. Click to enlarge.
Acetylene can be liquefied and solidified, however, both in the gaseous state at pressures above about 7 bar, and in the liquid and solid states, acetylene is shock sensitive and explosive. Therefore, acetylene is always supplied to users in cylinders dissolved in acetone or dimethylformamide and completely filled with a porous filler Agamassan (or AGA-massan, which stands for “AGA composition” in Swedish. AGA is the name of a Swedish manufacturer and supplier of industrial gases , now a division of Linde Gas, founded at one time by the inventor of Agamassan, Gustaf Dahlen. Agamassan's composition includes asbestos, cement, coal and kieselguhr). As an alternative to Agamassan, filler based on kieselguhr or ceramics/lime silicate can be used.
The excess pressure in acetylene cylinders is usually no more than 17 bar, and the outlet pressure from the cylinder is no more than 1 bar, and usually about 0.5 bar.
Acetylene cylinders are usually equipped with both conventional safety valves that operate when the pressure rises, including passing and isothermal, and special safety valves that operate when the temperature rises above 100°C, releasing acetylene into the atmosphere. Such valves act like fusible links.
In Russia, acetylene cylinders are painted white, with the red inscription “Acetylene”.
Notes
- ↑ 12
GOST 5457-75. Acetylene dissolved and gaseous technical. Specifications - ↑
A.L.Lapidus, I.A.Golubeva, F.G.Zhagfarov. Gas chemistry. Tutorial. - Moscow: TsentrLitNefteGaz, 2008. - 450 p. — ISBN 978-5-902665-31-1. - ↑
Big encyclopedia of oil and gas. Unpleasant odor - acetylene. Retrieved October 10, 2013. - ↑
Korolchenko, Fire and explosion hazard of substances, 2004, p. 198. - ↑
Miller. Acetylene, its properties, production and use, 1969, p. 72. - ↑
Acetylene. Retrieved October 10, 2013. - ↑
Russia has developed an ammonia rocket engine - Izvestia
Links
- Acetylene // Encyclopedic Dictionary of Brockhaus and Efron: in 86 volumes (82 volumes and 4 additional). - St. Petersburg, 1890-1907.
| Hydrocarbons | |
| Alkanes | Methane • Ethane • Propane • Butane • Pentane • Hexane • Heptane • Octane • Nonane • Decane • Undecane • Dodecane • Tridecane • Tetradecane • Hexadecane • Heptadecane • Octadecane • Nonadecane • Eicosane • Docosane • Hectane |
| Alkenes | Ethylene • Propene • Butenes • Pentenes • Hexenes • Heptenes • Octene |
| Alkynes | Acetylene • Propyne • Butine |
| Dienes | Propadiene • Butadiene • Isoprene • Cyclobutadiene |
| Other unsaturated | Vinylacetylene • Diacetylene • Carotene |
| Cycloalkanes | Cyclopropane • Cyclobutane • Cyclopentane • Cyclohexane • Decalin • Indane • Indene |
| Aromatic | Benzene • Toluene • Dimethylbenzenes • Ethylbenzene • Propylbenzene • Cumene • Styrene • Phenylacetylene • Indane • Diphenyl • Diphenylmethane • Triphenylmethane • Tetraphenylmethane • Indene |
| Polycyclic | Naphthalene • Anthracene • Benzanthracene • Pentacene • Phenanthrene • Pyrene • Benzpyrene • Azulene • Chrysene |
acetylene, acetylene + water, acetylene Wikipedia, acetylene Wikipedia, acetylene gargan avah, acetylene Donetsk, acetylene production, acetylene formula, acetylene chlorination, acetylene generator