AND
Research by RAS professor, professor and head of the materials design laboratory at the Skolkovo Institute of Science and Technology
Artem Oganov
is focused on the use of computational methods in the creation of new materials, studying the behavior of matter at high pressures and the development of new computational methodologies for predicting the structure and properties of materials.
In 2006, together with his student Colin Glass
he created a new method called USPEX (Universal Structure Predictor: Evolutionary Xtallography), which allows one to calculate the structure of a mineral for a given temperature and pressure, based only on the chemical composition. This unique evolutionary method of crystal structure prediction is currently used by more than 4,500 researchers worldwide. This method allows the prediction of new materials, both organic and inorganic, with desired properties and has enabled numerous discoveries in both basic and applied research.
We started our meeting with Artem Romaevich with the question:
— Are you studying, if I formulate correctly, the structures of crystals under different conditions?
— We predict the structure of matter. We predict a structure that is stable under certain conditions. We also predict the structure and composition of those materials that have the properties we need.
— That is, you set any desired properties in a computer algorithm and from this result you unwind the process in the opposite direction?
- Yes, that’s right - we solve inverse problems.
— Do you have competitors in this niche?
— When we started, there were few competitors. Now many people are trying to do this with various successes or failures, but we still hold the palm, and our method is still unbeatable in terms of reliability and speed. And by the number and variety of functions it has.
Structure of the transparent form of sodium
"Forbidden" chemistry
— You often say that you are involved in “forbidden chemistry.” Why is it banned?
— There are rules of classical chemistry that allow the existence of some compounds and prohibit the existence of others. These rules work quite well for a lot of connections. For example, we know that nothing other than NaCl is allowed by classical chemistry for sodium chloride. Sodium chloride is always NaCl. But it turns out that if you go to unusual conditions, such as high pressures, you can get stable crystals of the composition, for example, Na3Cl. This is prohibited in classical chemistry.
In fact, we don’t fully understand why they are formed, but they are formed, that’s a fact. Many of them are metals, and for metals these kinds of rules do not apply. But why they turn out to be metals is also unclear. Because, again from the point of view of classical chemistry, there must be an ionic bond between the Na and Cl atoms, where electrons from the Na atoms completely flow to the Cl atom and are strongly localized there. There shouldn't be a metal connection here. But nevertheless, for some reason it occurs at pressures, even not very high.
— Some physicists believe that everything in chemistry can be explained by physics. That ultimately it all comes down to solving the Schrödinger equation. How do you feel about such statements?
— There is a big difference between the words “explain” and “calculate.” Or “explain” and “predict”. Our predictions and calculations are truly based on the laws of physics, on quantum mechanics, on the solution of the Schrödinger equation or its analogues. But the calculation itself does not provide an explanation. The calculation will predict that at such and such pressures the Na3Cl compound becomes stable. Why is it stable? No answer.
Quantum mechanics operates at a very detailed level. At the level of nuclei and electrons. After all, in chemistry we are accustomed to operating at the level of atoms, each of which has some kind of character. Some atoms are active, some are passive, some atoms like to give up electrons, some like to accept. Some people don’t like to give or receive.
We predict the structure of matter. We predict a structure that is stable under certain conditions. We also predict the structure and composition of those materials that have the properties we need.
If sodium donates an electron, it will have a charge of +1. And if chlorine takes an electron, it will gain a charge of −1. But electrical neutrality must be observed. And we immediately come to the conclusion that only one NaCl compound is possible in a proportion of exactly 1:1, otherwise your balance of charges will not converge. Why does Na3Cl exist then? Should not.
But quantum mechanical calculations confront us with a fact based on solutions to the equations of quantum mechanics. Before the fact, Na3Cl will be stable. But how to explain it from the point of view of the character and disposition of the Na and Cl atoms is not clear. From the point of view of the behavior of atoms known to us, such a substance should not exist.
The laws of physics determine the chemical behavior of elements. The chemical behavior of elements explains the biochemical role of certain molecules. And the biochemical role of certain molecules dictates all processes occurring in living beings. Yes, the laws of physics determine everything. But can the laws of physics explain, for example, what you are thinking about now? It would be quite naive to interpret human behavior on the basis of the Schrödinger equation. This is called reductionism, and it is very limited in its applicability. To explain human behavior, it is still better to use the knowledge about human character that psychological science gives us. And to explain chemical phenomena, we need to use the knowledge about the nature of atoms that chemical science gives us. Work in recent years shows that we do not understand something important in the character of seemingly well-known atoms.
Wins - composition and properties
It appeared in the USSR back in 1929. The material is a hard metal alloy. The composition of Pobedite includes tungsten carbide and cobalt, the mass ratio of which is 90:10.
Pobedit is characterized by high strength, wear resistance and hardness. However, products made from Pobedite exhibit such disadvantages as causticity and fragility upon impact, so in practice Pobedite spraying and the use of cutting and drilling tool tips are used. Thanks to the tips and spraying, the tool is given properties that significantly increase wear resistance to abrasion and dullness.
Manufacturing method
The alloy is produced in industry not by casting, like cast iron and copper alloys, but by sintering a pressed mixture of composite powder. A ceramic-metal composite alloy is created by mixing fine tungsten carbide powder and cobalt as a binder. The resulting fine powder mixture is placed in special molds in the form of a plate of various shapes and sizes and sintered. Due to the high sintering temperature, which is close to the melting point of the binder material, the result is a unique material with high strength and hardness.
Unlike classical technology, the main components of the alloy can be replaced with other analogues, for example, nickel can act as a binder. However, in practice these alloys are also usually called pobedit.
Scope of application
It is practically indispensable in the mining industry, where it is used as a spray coating for drilling parts of hard rock drilling equipment. In metalworking, pobedit coatings and tips are used in metal-cutting equipment and drawing tools. Pobeda also finds its application in woodworking. Pobedit's high hardness and heat resistance effectively protects equipment used in difficult conditions, giving it good wear resistance characteristics.
At the domestic level and in construction, drills for rotary hammers and impact drills are used for drilling concrete walls.
Our company will buy in any quantities from individuals and organizations.
Source of the article: https://drag-radiodetali.com/ru/stati/pobedit-svojstva-primenenie
Don't forget to practice
— Do you always look for practical applications for your developments?
— I was raised as a fundamental, not an applied scientist. Moreover, in my circle it was believed that a scientist should not think about the practical applications of his work, that this was a dirty form of science, unworthy of a thinking person. But over the years, I realized that this is an absolutely erroneous and even vicious approach.
There are scientists who are entirely fundamental; their discoveries are probably impossible to apply to practice. But they expand the boundaries of knowledge. Knowledge, for example, of black holes has no practical value. Although on the way to this knowledge people are improving their mathematical apparatus and instrumentation, they can be useful somewhere.
I was raised as a fundamental rather than an applied scientist. Moreover, in my circle it was believed that a scientist should not think about the practical applications of his work, that this was a dirty form of science, unworthy of a thinking person. But over the years, I realized that this is an absolutely erroneous and even vicious approach.
I remember my visit to Dubna, to the laboratory of nuclear reactions, which was headed by our great scientist Yuri Tsolakovich Oganesyan for many years. The man who discovered nine elements of the periodic table, one of which - number 118 - was named "Oganeson" in his honor. So they asked him: what is the practical benefit of these elements, which are synthesized in ultra-small quantities? He honestly answered that there was absolutely no practical benefit and never would be. But on the way to these discoveries, they created new accelerators. And these accelerators can be useful in the treatment, perhaps, of cancer, as well as for the creation of special membranes. And they have technology that they have been developing and using for decades. They take a polymer film and pull it in front of an accelerator at a speed of, say, a meter per second. During this second, a square meter of plastic film is subjected to intense particle bombardment. And these particles leave holes there. And you can use this perforated polymer film as a membrane, as a filter. For example, no bacteria will be able to pass through these holes, which opens up many applications. And this is just a side effect.
-
You need to be able to notice this scope of application.
- Don't forget that we are people. And in humans, the passion for knowledge is one of the basic instincts. We usually talk about the instincts of self-preservation and reproduction, but there are two instincts that also seem to me to be fundamental for humans. The first is the instinct of knowledge. A person is always interested: “how does it work?” Remember how many toys every child breaks, trying to figure out what's inside.
The second instinct is to create and change the world around you. This is described in the Bible with the words: “God created man in his own image and likeness.” Man is a creator. He loves to change the world in which he lives, he loves to create something new. This is our nature.
— The new knowledge that a scientist gives can greatly change a person’s practical life and the course of history in general. Does this instinct - to change the world, to leave a mark after yourself - prevail in you?
— I see in myself a very strong instinct to change something. Largely because of this, I returned to Russia. Because I realized that when the Skolkovo project was unfolding, I liked this idea of Skolkovo and Skoltech in particular. And I realized for myself that this is an opportunity for me to participate in creating a critical mass of brains here. And if I don’t take part in this, I will never forgive myself for this. I will never forgive myself if this project is successful and it turns out that I have no role in it, because I chickened out and didn’t come. And if this project is not successful, I won’t forgive myself either - after all, if I had come, maybe this would be that very drop that would be enough for the project to become successful.
Structure of a substance with the formula Na2He
— If you are focused on broad practical applications, you can make a business out of your discoveries?
“That’s exactly why my brother and I created a company.” Now she is just at the start. We give the USPEX program, which I created, to scientists free of charge. But we sell it to companies. Sony, Toyota and others pay quite a lot of money for the program. And each of the main authors of this program, me, my students, owns some percentage of these sales. I have already bought a house with my percentage.
— For what purpose
Toyota use your program?
What are they looking for? — As far as I understand, they are looking for materials for lithium batteries. I see from their publications that they are interested in this particular topic. But besides publications, there are also internal results, they are confidential, and I don’t even ask about them.
Sometimes you can even become a completely wealthy person without leaving science if you can find something that changes the life of mankind. I can give a wonderful example of a Chinese scientist from the Institute of Coal Chemistry of the Chinese Academy of Sciences. He worked in Germany for some time; of course, he was extremely poor. But a happy idea came to him, and he invented a new catalyst, which greatly reduces the energy intensity of the coal gasification process and makes it possible to create high-quality gasoline and diesel that do not contain sulfur from dirty coal. If you can create a catalyst better than what was created before you, you can run this process much more economically and efficiently. So he returned to China and in a short time became an extremely rich man. Given the relatively high price of oil, its production is absolutely profitable. And who would have thought that this rich man, a famous professor in China, just recently, some fifteen years ago, hardly had a penny to his name. He now has his own estate near Beijing, his own horses, his own restaurant, to which he takes guests. I was deeply impressed when I saw all this with my own eyes.
Pobedit is the general name for hard alloys of tungsten carbide and cobalt.
The name “will win” arose during the Battle of Moscow (1941), due to the fact that armor-piercing bullets for 14.5 mm anti-tank rifles were made from the alloy. There is another version of the origin of the name, which suggests that the name originated in military factories in the rear, where without such an alloy it would not have been possible to produce the necessary amount of weapons for the front.
Tools based on sintered tungsten carbide began to be used in the mid-1920s in Germany. In the USSR, Pobedit was developed in 1929, where it was mainly used for cutting tools.
Initially, tungsten carbide and cobalt in the winning alloy had a ratio of 96:4 by weight. Currently, other tungsten-cobalt composite alloys have been developed, but the name “win” continues to be used for them.
The winner will win
Well and tunnel builders around the world are looking for a complete and yet economical replacement for the expensive diamond and pobedit cutters used on drilling equipment. Fundamental science, in turn, has been struggling for decades to find new compounds and alloys not found in nature. Read about how Russian oil workers helped scientists make a discovery that could lead to the industrial production of a new superhard material - tungsten pentaboride.
Jewelry tool
Diamonds have been drillers’ best friends for a century and a half. In 1863, engineer Rodolfo Loscher first used the prototype of the modern diamond bit during the construction of a railway tunnel in the Swiss Alps.
The steel drills that were used then failed after just an hour of operation. According to legend, in despair watching the futile attempts to break through the mountain, Losche tapped his fingers on the window glass and noticed the traces of a diamond ring remaining on it.
Despite the crazy high cost (the cost of one carat of diamond in the mid-19th century was comparable to the cost of a horse-drawn carriage), Losche was able to persuade the investor to purchase 100 carats of diamonds. The precious stones were attached to the drill pipe manually: a separate socket was drilled at the end of the drill for each crystal and filled with special solder.
Soon the work began to boil. The diamonds crumbled, fell out of their nests, some of them probably never returned to their place, having rolled into the pockets of the workers, but still the precious drills paid off: the speed of penetration accelerated tens of times - instead of an hour, they were enough for a day.
Today, one diamond cutter costs between $20 and $200. Drill bits come in different designs: on average, they have 50 cutters, so the cost of the tool varies from half to several million rubles. The service life strongly depends on the composition of the rock that the drillers “gnaw”: in the conditions of Eastern Siberia, one bit travels 200-500 meters, and in Western Siberia - 10 kilometers or more.
It doesn't get any harder
Can anything replace a diamond? The question of whether a harder substance exists is of great interest not only to drillers, but also to the scientific community.
Over decades of searching, hundreds of publications were published, the authors of which claimed that they had finally found, or at least understood, where to look for a structure comparable to diamond, or even superior to it in hardness. All these allegations were subsequently invariably refuted.
So far, no known substance can compete with diamond in this property. But it has its drawbacks - in an oxygen atmosphere, diamond begins to burn at a temperature of 1000 degrees Celsius, and at higher temperatures it “dissolves” in iron-containing rocks.
A decade ago, Chinese scientists said that, according to their calculations, in the absence of impurities, the mineral lonsdaleite - a hexagonal polymorph of diamond first synthesized in the laboratory in 1966 - could be 58 percent harder than diamond. However, these theories have never been confirmed.
The search for a material that will replace the diamond cutter insert continues. Russian scientists have already obtained samples of new superhard materials, which in their characteristics are very close to such a substance as cubic boron nitride. This is one of the superhard compounds closest to diamond used in industry.
Crystal structures of superhard materials
Oganov et al. Journal of Applied Physics, 2019
Share
Will he win all?
Until the end of the 19th century, the underground drilling and mining industries used only carbon-infused tool steel to create drill bits. Next came the idea of using a tungsten-carbon alloy for cutting tools. It was first used in the 1920s at Krupp factories in Germany.
In the USSR in 1929, the “canonical” ratio of tungsten carbide and cobalt in the alloy was patented - 9 to 1. Soviet engineers called the alloy, quite in the spirit of the times, victorious. Today there are already dozens of winners: many include not only tungsten and cobalt, but also nickel, titanium, and tantalum.
A pobedite drill drills into concrete and can even pierce metal in it. Such drills cope with work on hard soils and rocky rocks.
For decades, cutter heads for drilling rigs all over the world have been made from pobedite (tungsten carbide) interspersed with synthetic diamonds. They are beyond competition in the market; other materials have not been able to supplant them.
Even harder materials, such as titanium diboride, either require high pressures during their synthesis, and therefore have a high cost, or have much lower crack resistance and are less practical to use.
Between tungsten and boron
In 2015, Russian oil workers and scientists from Skoltech decided to unite in order to jointly obtain a material capable of winning.
“At some point we asked ourselves,” recalls Artem Zakirov, an expert at the Scientific and Technical Institute, “whether it was possible to use a different material for drilling cutters that would be more wear-resistant and would not require high pressures during synthesis.”
The answer to this question was sought between tungsten and boron. It is known that they can form among themselves many stable crystalline phases of different compositions: two phases of composition WB and three more compounds WB2, W2B, WB4.
In the course of new research, crystallographers have discovered three more stable structures that were previously unknown: tetratungsten triboride (W4B3), hexatungsten pentaboride (W6B5) and tungsten pentaboride (WB5). All three phases turned out to be refractory and superhard, and scientists called tungsten pentaboride WB5 the most interesting of them.
According to calculations, the hardness of pentaboride is at the level of 45 gigapascals. And its properties must be maintained even at very high temperatures - for example, the hardness of the new material when heated to 2000 degrees Celsius drops only to 27 gigapascals. At the same time, for example, the diamond would already be blazing with a blue flame.
Gazprom Neft was the first to test prototypes of cutters for drilling equipment made from newly designed materials. Tungsten pentaboride was tested on granite. The test confirmed that the samples are harder than Pobedit and its analogues. The unique material turned out to be 30 percent stronger and 2 times more resistant to high temperatures.
Now Gazprom Neft continues to explore ways to produce new materials and products based on them using industrial equipment. Especially for this purpose, together with the Russian Science Foundation, the company opened a laboratory for computer design of new materials at Skoltech.
Gazprom Neft
Share
Evolution of success
“The easiest way to interact with business is when you are asked to solve a problem for which you have already half-thought out the solution,” says Artem Oganov, a Russian crystallographer and professor at Skoltech. “We have been studying many systems for a long time, predicting stable chemical compounds and calculating their properties. These were interesting substances, but they were not comparable to pobedit in terms of hardness. It seemed that the winner was truly invincible.”
Oganov's weapon is USPEX. Read this abbreviation however you want, but it stands for Universal Structure Predictor: Evolutionary (X)Crystallography. It is a machine algorithm for predicting crystal structures. It predicts what stable structure a substance with a given chemical composition will have under certain conditions.
The most stable structure of a substance has the least energy. In this case, energy characterizes the electromagnetic interaction of the nuclei and electrons of the atoms that make up the crystal. It is practically useless to search for structures with the lowest energy by simple search: even if the system consists of only a dozen atoms, there will be about 100 billion options for their arrangement relative to each other.
USPEX randomly generates a small number of structures and calculates their energy. And then evolution begins in the literal sense of the word: options with the highest energy, that is, the least stable structures, are discarded, and the algorithm generates derivative structures from the most stable ones. If their energy turns out to be lower than the “mother’s”, but the next “generation” is produced from them.
Reduce pressure
New superhard materials were sent to the Vereshchagin Institute of High Pressure Physics of the Russian Academy of Sciences to verify the results of Skoltech scientists.
“We had experience working with borides, accumulated over the previous 30 years, but we did not explore areas of higher boron concentrations, since such alloys are more brittle,” says Vadim Brazhkin, director of the Institute of Physics and Physics of the Russian Academy of Sciences.
In a chamber with a maximum pressure of 15 thousand atmospheres (approximately corresponding to 15 kilobars), prototypes from tungsten pentaboride several millimeters long were synthesized at the Institute of High-Property Physics. Larger incisor prototypes are not required since the working elements of the incisor crowns do not exceed 15 millimeters. In terms of mechanical indicators, samples obtained at high pressure are superior, but lose in terms of cost.
The IFVD explains that, according to their calculations, for implementation on an industrial scale it is necessary to learn how to synthesize pentaboride crowns at pressures less than 10 kilobars. The institute is now actively working on this. If successful, scientists will have to find a suitable site for production, convince service companies of the benefits of introducing the new material and protect patent rights.
“We wanted to build a technological chain from fundamental science to practical application. In our country, this institution of transfer and business requests to fundamental science is not developed. Using tungsten pentaboride as an example, we are trying to create it practically from scratch,” Gazprom Neft admits.
"Treasure map"
And while pentaboride was being baked and tested at the Institute of Higher Physics, theorists continued their search. And in a new publication, Oganov and colleagues from Skoltech and MIPT described a combination of the USPEX algorithm with two new methods for calculating Vickers hardness and impact toughness (the ability to absorb energy without destruction).
After ruling out noble gases, rare earth elements and radioactive nuclides, the scientists tested binary combinations of 74 elements from the periodic table. The result of their work was a “treasure map” of superhard materials, which identifies both already known and new substances of varying degrees of hardness and toughness.
The “map” presents both well-known materials: tungsten carbide, corundum, and promising ones. One of the new marks on this “treasure map” is tungsten pentaboride.
Crystallographers have also discovered superhard qualities in manganese hydride, a material that had never before been studied as a superhard phase. However, it turned out to be harder than stishovite, a super-hard silicon oxide created by meteorite impacts.
Nikolay Kozin
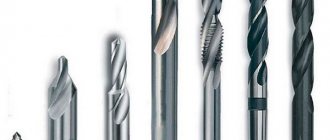
Pobedite drill: types, application, sharpening, features
When working with materials such as wood, plastic and non-alloy steel, conventional twist drills are used. Tool steel is usually used for their manufacture. But when you have to work with materials with high hardness, a standard cutting tool may not cope with the task and may even break. Common materials such as stone, reinforced concrete, brick and durable high-alloy steel alloys have a much higher hardness than conventional cutting tools. Therefore, to process them you need to use drills made of durable carbide alloys. One of the most suitable options for such a tool is the alloy that will win. Pobedite drills are characterized by high hardness and can drill holes even in the most difficult materials. Let's look at what a Victory drill is and how to work with it correctly.
Nonlinear brain
— A happy idea and hard work, discipline, as you say, together are capable of a lot.
— The human brain works very nonlinearly. Sometimes he needs the utmost degree of tension to answer a question. And sometimes you just need to give it a rest.
We give the USPEX program, which I created, to scientists free of charge. But we sell it to companies. Sony, Toyota and others pay quite a lot of money for the program. And each of the main authors of this program, me, my students, owns some percentage of these sales. I have already bought a house with my percentage.
A lot of ideas come to me, for example, when I’m taking a steam bath. I really love the bathhouse. This, by the way, was also one of the reasons why I returned to Russia: I can’t exist for long without a bathhouse. I lived on Long Island, and to go to the bathhouse required me to travel three to four hours each way. There are other purely Russian things that I really missed in the West, for example, sea buckthorn and sea buckthorn jam. But, of course, these are secondary reasons. By the way, when I returned, already four years ago, there was a wonderful bathhouse company of my close friends. I think we have the most amazing bath company in the world, and its IQ will be greater than that of the entire US Congress. There is my childhood friend Pavel Plechov, director of the Mineralogical Museum, professor at Moscow State University. In the same company is Viktor Enin, who created a network of wonderful tea houses in Moscow. Literary critic and exceptionally unconventional person Dmitry Itskovich, who, in order to create a very advanced publishing house, created a chain of restaurants. Professor of Oriental Studies Alexey Muravyov, who as a child starred in the film “Guest from the Future”: he speaks half of the European languages, plus Georgian, Armenian, Aramaic, Arabic, Coptic, Ethiopian.
And this is probably the whole essence of the concept of happiness. Find a job that you like and find people with whom you want to live, communicate, and be friends.
— How do you assemble your scientific teams? On what basis?
“Every person needs to be in his place.” But first of all, you need to hire talented people into your team. I try to take people who are superior to me in at least some way.
I was lucky with my students; in total, they turned out to be twelve professors. At the same time, the two best students seemed rather average to me at first.
— What are the “best” students?
- When you thought you knew this person’s limit, but he takes it and jumps higher. And you think: okay, now I definitely know the limit of this person. And then he takes it and jumps even higher. And when this happens for years, you begin to understand that you don’t know the limit of this person. And maybe he doesn't exist.
What does it mean to win?
Engineers have been developing especially hard metal alloys for the manufacture of cutting tools since the beginning of the development of mechanical processing of metal on an industrial scale. And by the twenties of the last century, foreign and domestic scientists were able to find the optimal alloy composition that would satisfy the stated needs. This alloy was named Pobedit. Pobedit is a name given to a whole range of metal alloys with increased hardness, strength and wear resistance.
According to the Rockwell method, hardness indicators should reach 85-90 units.
The main material in Pobedite is tungsten carbide. Its content is within 90%. The remaining important components are cobalt and a limited amount of carbon. In some cases, expensive tungsten has recently been replaced with titanium, but if replaced, the alloy slightly deteriorates its important characteristics. Cobalt can also sometimes be replaced with nickel. Pobedit is a heat-resistant material. Its melting point is 3150 ˚С. It begins to lose its properties and become soft only at 1200˚C. They are obtained mainly by powder metallurgy methods.
Pobedit includes such grades of hard alloys as VK4, VK10, T15K6, V8, V6 and many other new alloys.
The disadvantages of this material include high fragility and low impact resistance, as well as relatively high cost. Therefore, metal-cutting tools are not made entirely of pobedit, but only some parts of drills are made from it or sprayed onto the surface. A pobedit drill has a cutting edge made of pobedit, and therefore has high strength and hardness.
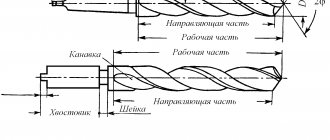
Design features of a drill with pobedit
A pobedite drill does not differ significantly in appearance from a standard twist drill. It also consists of a shank, a working part and a cutting edge and has a cylindrical shape. The shank can be cylindrical, hexagonal, or have an SDS profile if it needs to be installed in a hammer drill. The working part of the drill is made of tool steel and has a number of helical grooves on the surface designed to remove chips from the drilling zone. But the drill tip is made of hard alloy - pobeda.
Drills with a Pobedit tip do not require pre-sharpening immediately after purchase. They are ready for use, and their cutting profile angle is 130 degrees. The pobedite tip usually has a flat shape. Its width is identical to the diameter of the hole that needs to be made in the workpiece. This tip is fused to the working part of the tool. The tip of the drill is the center of the tip. The tip divides it strictly into two equal parts. If they are not equal, then such a tool will not work.
Is it possible to sharpen a Victory bit?
When dull, a drill bit with pobedit tip can be sharpened. The sharpening angle depends on the hardness of the material that needs to be drilled. For the hardest material, the smallest possible angle should be provided. The sharpening process must be done carefully so as not to destroy or overheat the pobedit soldering. To do this, you can cool it.
Typically, a drill with a Pobedit tip has a diameter of 3 millimeters. The maximum diameter of drills for hand tools is 12 millimeters, but can be larger.
The length of the drill ranges from 70 to 200 millimeters and increases with increasing diameter.
What are drill bits with pobedit used for?
For proper use, you need to know exactly what the Pobedit drills are intended for. After all, if you use them to drill inappropriate material, you will not only not achieve your goal, but also destroy a very expensive tool.
The basic rule for using a drill with a winch is the fact that it can only process material that has less hardness.
The main area of application of such drills is the processing of concrete, brick, artificial and natural stone, marble, granite, and ceramic products. All these materials have high hardness and cannot be processed using conventional drills. Pobedit concrete drills are designed for installation on a hammer drill, and therefore work well in conditions of not only cutting, but also impact.
Concrete and stone are hard but fragile materials, so after work, a lot of stuck waste remains on the drill, which must be removed.
When drilling deep holes in durable material, it is advisable to cool the Pobedit concrete drill. If operated at high speed for a long period of time, the weld bead may be damaged or even separated from the drill body.
Periodic cooling can be done with ordinary water, but it should be taken into account that if the cooling is too rapid, the solder may burst.
The operating principle of a Pobedit drill is based on chipping the material being removed rather than cutting it. Therefore, it is not suitable for working with all types of materials. But special pobedit drills for metal are produced. They are similar in design to other Pobedit drills, but may differ in the sharpening angle of the cutting edge and have additional coating. When working with such a tool, intense heating occurs when metal rubs against metal, so Pobedit metal drills require very active cooling.
Between the material and immaterial world
— Why do you do science?
- Because it interests me. And because I probably don’t know how to do anything else.
-Have you tried it?
- I didn’t even try. All my life I dreamed of being a scientist and only a scientist. And it never even occurred to me that I could become someone else. With the exception of one period in my youth when I thought about becoming a priest. And at some point I almost became one.
— An interesting balance between this area of knowledge and, let’s say, science that requires evidence.
Structure of metallic sodium chloride NaCl3
- They complement each other. These are different aspects of knowledge of the world, the material world, which is studied by science, and the intangible world. If people operate only in material concepts, then I am afraid that they will not advance far in their development.
Religion is one of the ways of education. Science will not tell you what is good and what is bad. Dostoevsky wrote: “If there is no God, then everything is permitted.” In a purely materialistic picture of the world there is no absolute coordinate system, no absolute criterion of good and evil.
And it is necessary to know what good and evil are. If everything is permitted and you do not introduce restrictions into your life, or any taboos for yourself, then this will not lead to anything good. Without internal discipline a person will not advance far. Each of us has probably encountered a huge number of talented people, of whom nothing came of it as a result. Only because they did not have this inner discipline or what we would call the core.
—What is important to a scientist?
— In science, the most important thing is to be a good scientist. I think that science is one of those aspects of human life where there is a mechanism for proving that you are right, and being right in science does not depend on your regalia or administrative resources.
Yes, the laws of physics determine everything. But can the laws of physics explain, for example, what you are thinking about now? It would be quite naive to interpret human behavior on the basis of the Schrödinger equation.
No one doubts that the geneticists were right and Lysenko was wrong. Why? Because there is experimental evidence that genetics works.
A scientist is a person who seeks truth, not profit.
And truth does not depend on place, time or political situation.
“However, recently there have been a lot of fakes.
- This is a huge problem, a problem in many ways of educating people, a problem of society. Rich countries have a lot of scientists and fierce competition. And only those who can loudly declare themselves and produce some kind of loud result get ahead. But this is no excuse for wanting to invent discoveries that do not exist. There was a recent story in Japan when one lady made a brilliant career. A hot topic - stem cells, high-profile results, plus a woman in science - this is still a symbol. And so she became a national symbol, and then it turned out that her scientific results were fake. And it turned out to be a disaster, the end of her career, the suicide of one of her superiors and a stain on the reputation of the entire institute. In Russia, this problem also exists, but, rather, because of the scientific hierarchy, those same bosses who are always right. And their subordinates get the result that will confirm the boss is right, and the more the boss is wrong, the more the subordinates have to force numbers, facts and common sense in order to earn his approval.
Advantages and disadvantages
The distinctive properties of the pobedit tool made it indispensable when carrying out a variety of construction and repair work.
Among the obvious advantages of Pobedit drills, it is important to note the following properties:
- These drills can successfully process even hard materials such as brick, concrete, ceramic tiles, marble and granite. Regular tool steel drills will not be able to handle this.
- Thanks to its thoughtful design, a Pobedit drill will not be much more expensive than a regular drill without an additional tip.
- The tool does not require preliminary sharpening and is immediately ready for use.
- A large number of types of Pobedit drills are produced. Therefore, finding a tool with a shank for the required type of chuck is not difficult.
- Pobedit takes shock loads well, so it works successfully on a hammer drill in shock mode.
- Some high-quality drills are capable of processing reinforced concrete by drilling through reinforcement.
- Pobedit is not afraid of abrasive inclusions, which are found everywhere in concrete.
The disadvantages of this tool include the following:
- Since the drill crumbles the material being processed, it is almost impossible to get high-quality holes in metal or wood. To process them, it is better to use a different type of tool.
- The cost of a Pobedit drill will be slightly higher than usual, but this is justified by their uniqueness, durability and performance.
- When working with particularly durable materials, Pobedit concrete drills require periodic cooling.
- When using a cheap tool, pobedite deposits often burst or become unsoldered from the drill.