The Sendzimir method, named after the Polish engineer and industrialist who developed it, is by far the most common method of applying a thin layer of zinc to steel. Meanwhile, the question of whether this method is capable of providing reliable protection for steel products used outdoors is asked by many of those who are planning to use this technology.
The Sendzimir method is used in continuous galvanizing plants for coiled steel
Production stages
While the technology developed by Sendzimir Tadeusz is considered quite simple and effective, the process of applying protective zinc coating is quite complex. Steel processing occurs in stages and involves the continuous immersion of appropriately prepared elements into the molten composition.
- Metal sheets up to 3mm thick are thoroughly cleaned with reagents, the purpose of this action is to obtain a perfectly clean surface. Then the sheets are dried and heated in special installations at t = 650C.
- The steel prepared in this way is immersed in liquid zinc poured into special baths. At this time, a complex set of diffusion processes occurs, as a result of which a protective zinc barrier is formed on the surface of the sheet.
- The metal removed from the container is subjected to treatment with compressed gas supplied under pressure using special devices with nozzles called “gas knives”. In this way the sheets are cooled while the thickness of the coating is perfectly adjusted.
Processing steel using a method invented by a Polish engineer makes it possible to achieve the formation of a homogeneous, thin and at the same time dense impermeable barrier, up to 20 microns thick, with a density of 275 g/m2. In this process, zinc is inextricably bound to the iron alloy, and its atoms penetrate into the metal sheet.
This is interesting: Centering drills - characteristics, application, GOST
Efficiency of galvanizing
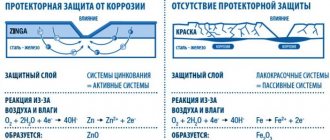
The most common form of corrosion is a reaction with oxygen and water present in the air. This natural reaction is often called oxidation. Zinc safety fencing, as its name suggests, provides an impenetrable barrier to a steel object.
Galvanizing forms defense in three directions:
- Creates effective mechanical protection as the coating is extremely resistant to damage, abrasion and scratches.
- When exposed to corrosion, zinc “sacrifices” itself for the benefit of steel. This phenomenon is known as cathodic protection.
- As zinc oxidizes, it forms a new protective layer.
Let's take a closer look at the benefits of galvanizing.
Most important is the ability of zinc to create electrochemical protection. Its electronegativity is less than that of iron. In the presence of moisture, a spontaneous reaction occurs between the chemical elements, which effectively prevents the destruction of the previously produced alloy. Oxidation of zinc leads to the formation of new compounds, such as oxide and hydroxide, and these, in turn, form new strong armor.
Thanks to a unique electrochemical process, even if the coating is damaged, the steel product remains protected: new connections cover the damaged area. Cutting, perforating, drilling and bending do not lead to progressive rusting of the iron.
The process of corrosion of the zinc layer occurs quite slowly, and its “sacrificial” protection will work until it completely becomes unusable. That is why the service life of the coating is determined by the annual reduction in protective armor.
It is important to understand that the durability of the anti-corrosion barrier depends on its thickness. After processing steel using the Sendzimir technology, a filigree protective layer is formed, so the products have much less corrosion resistance than those protected by thicker galvanization.
Protective properties of the zinc layer
Steel coated with zinc using the Sendzimir method receives not only barrier protection, but also electrochemical protection. As a result of applying such a coating, a galvanic couple of zinc and iron is formed, and the charge of zinc eliminates the possibility of chemical reactions with iron. Due to this, it is not steel that is subject to corrosion, but zinc, and for this metal, such a process, as is known, proceeds much more slowly. Thus, the surface of the steel product will be safe until the zinc layer that is applied to it becomes unusable. Among specialists, such electrochemical protection is often called “sacrificial”.
The great advantage of galvanizing steel using the Tadeusz Sendzimir method is that even when cutting and drilling, products that have undergone such treatment are not subject to further corrosion. This is explained by the fact that at the site of damage to the protective layer, under the influence of oxygen and moisture, zinc hydroxide is formed, which also has decent protective properties.
Various products are made from sheet steel coated using this method, and damage to the zinc coating due to cutting or stamping does not cause progressive corrosion
Meanwhile, due to the fact that the zinc layer obtained by the Sendzimir method is not very thick, it is not recommended to use the products on which it is applied outdoors. A protective coating, the thickness of which does not exceed 20 microns, can quickly collapse not only under the influence of precipitation, but even from too high humidity. That is why galvanized products processed using the Sendzimir method should be used in dry rooms. Metal galvanized using this technology has a lower degree of corrosion resistance than hot-dip galvanized metal.
Cable trays for use outside of aggressive environments - an example of products manufactured using the Sendzimir method
Assessment of the quality of finished products
The quality of the coating using the Sendzimir method must comply with GOST 14918-80. This standard regulates products of the highest and first categories.
Coverage parameters are determined by the following indicators:
- density of the zinc layer – 275 g/m2;
- protection thickness – from 10 to 20 microns;
- shade varies from silver to matte gray;
Galvanized steel is intended for use in environments with corrosion class C1, C2 (use of products in dry rooms is recommended).
So, the Sendzimir method is deservedly considered a cost-effective way of applying protection to metal products, but it is used for those products whose operation will take place in comfortable conditions. It is recommended for use inside heated buildings with a clean atmosphere, such as shops or offices, as well as in unheated, dry areas such as gyms and warehouses.
How the process works
Galvanizing using the Sendzimir method involves immersing rolled metal, pre-heated in hydrogen, in molten zinc. The process proceeds in the following order:
- Submission of a batch of blanks. In mass production, raw materials are unrolled and fed continuously.
- Degreasing the surface to be treated. Parts are cleaned in a chemical bath filled with a solution of special reagents.
- Passing furnaces with an oxidizing atmosphere. The method was invented in the 40s of the last century. But it is still relevant today - unlike other galvanic processes, it does not require the use of toxic gases and substances.
- Being in a furnace with a reducing atmosphere (hydrogen + nitrogen). Partial saturation of the top layer of the workpiece makes it possible to achieve greater uniformity of the coating and enhance anti-corrosion properties.
- Partial cooling. The formation of parts from galvanized sheet occurs after the workpiece has undergone a galvanic process. Partial cooling is necessary to create optimal conditions for joining the steel and zinc layers.
- Galvanization in a bath of molten zinc at a temperature of about 460 ° C. Preliminary preparation of the workpiece makes it possible to connect the layers of iron and zinc at the molecular level, ensuring maximum coating strength.
- Drying. After leaving the bath, the material is sent to the oven, where the thickness of the coating is adjusted.
Electrolytic galvanizing (galvanic zinc coating)
Products are made from untreated sheet steel. After manufacturing, the products are pre-treated and immersed in a bath of zinc electrolyte.
A layer of zinc about 10 microns thick covers the entire product, including cut edges and welds.
Hot-dip galvanized products are suitable for use in premises classified in terms of environmental exposure to categories C1 and C2: dry rooms and open air without harmful and aggressive impurities.
Often referred to as EC.
Description of the technological process using the Sendzimir method
The Sendzimir galvanizing method involves drawing steel sheets through a zinc bath, resulting in a uniform zinc coating with a density of 275 g/m
2
and a thickness of approximately 10-20 microns, as well as with corrosion resistance according to classes C1-C2 (use in heated and unheated rooms). Regulated by GOST 14918-80.
Galvanizing stages:
- The preparatory stage at which the steel is treated with reagents and heated to 650°C.
- Coating steel with a layer of zinc by drawing steel sheets through a bath of molten zinc.
- Cleaning the surface of the steel from unnecessary zinc residues using compressed air, also compressed air helps to cool the galvanized steel sheet faster.
Steel galvanized by the Sendzimir method has a thin zinc layer (compared to hot zinc, hot zinc) but is strong enough to perform its protective functions. This method of metal processing allows cutting and drilling; in the exposed areas, the protective coating is not lost, due to the fact that zinc hydroxide, which has good anti-corrosion properties, is formed in these areas.
However, due to the thin protective layer, products made of galvanized steel using the Sendzimir method cannot be used outdoors; they can only be used in dry rooms. Therefore, despite the cost-effectiveness and speed of processing of this method, it should be chosen only for the specified climatic conditions.
Inventor of the method
Tadeusz Sendzimir is a Polish inventor in the field of metallurgy and mining, has more than 120 patents, many of his inventions are used in industries around the world. His most famous invention was a method of treating steel that protected it from corrosion. Has many awards and achievements. One of the largest metallurgical plants in Krakow bears his name.
This is interesting: Submerged arc welding - nuances of technology, advantages and disadvantages
Stainless steel and acid-proof steel
In this case, the steel is not subjected to additional processing, since it already has increased resistance to corrosion.
Products made from stainless and acid-resistant steel are used in highly aggressive environments in which hot-dip galvanizing is not sufficient protection of steel against corrosion. Products made of stainless and acid-resistant steel are suitable for both indoor and outdoor use.
The products can be used in areas classified as environmental exposure categories C3, C4, C5-I and C5-M. Typical applications for the products are chemical and wood processing industries, as well as facilities with increased hygiene requirements - dairy farms, slaughterhouses, food and pharmaceutical industries
Whether stainless or acid-resistant steel is used depends on many different factors. The factor that has the greatest influence on the choice of material is the chemical composition of the environment, i.e. the compounds contained in it and their percentage composition. In general, it can be stated that acid-resistant material has a higher climatic corrosion resistance level and is suitable for industrial and humid atmospheres. The high content of chloride compounds also requires the use of acid-resistant materials.
Technological stages
Galvanizing using the Sendzimir method is performed in a special way: sheet steel, on the surface of which it is necessary to apply a protective coating, is pulled through baths of molten zinc.
Galvanizing steel sheets, performed using this method, includes several stages.
- The steel sheet, the thickness of which can reach up to 3 mm, is treated with special reagents and then dried in an oven at a temperature of 650°.
- The dried and heated steel sheet is fed into a bath that is filled with molten zinc. Passing through such a bath, the sheet is evenly covered with a zinc layer.
- At the exit from the bath, the steel sheet is exposed to so-called gas knives - devices through the nozzles of which compressed air is supplied to the surface of the sheet under high pressure. Due to this effect, two problems are solved at once: excess zinc is removed from the surface being treated, and it is also cooled.
The principle of galvanizing according to the Sendzimir method
The galvanizing method, named after its developer, Sendzimir, allows the formation of a dense and uniform zinc layer on the surface of a steel sheet. The thickness of such a protective layer, as a rule, is in the range of 10–20 microns, and its density is 275 g/m
2
.
Cold galvanizing method of metal
Galvanizing in this case is carried out using anti-corrosion compounds regulated by GOST 9.305–84. This standard describes the features of all commercially available inorganic coatings (including metallic ones) produced by chemical and electrochemical methods. In particular, it states that cold galvanizing can be used on any materials except products made of magnesium alloys and high-strength steel.
VT-metall offers services:
Immediately before applying the protective composition to the surface of the workpiece (in accordance with GOST), it should be prepared. Namely:
- remove dirt from the surface from atmospheric influences, salts and coking by thorough washing;
- clean the surface of the product using waterjet, abrasive blasting or hydrodynamic tools to give it the required degree of roughness, and in the case of an old coating, to remove residual rust and scale;
- after using a waterjet or hydrodynamic cleaning method, thoroughly dry the surface of the product;
- carry out manual finishing cleaning of the metal surface from welding spatter, burrs, sharp corners and edges;
- Dust the product with a jet of compressed air.
Recommended reading
- Cutting copper with a laser: advantages and disadvantages of technology
- Types of metal cutting: industrial applications
- Metalworking according to drawings: convenient and profitable
The quality of the procedures performed is then checked. After which you can apply the cold galvanizing compound. In accordance with the technology, the composition is applied at a certain temperature recommended by the instructions for this coating. Moreover, this temperature must exceed the dew point by at least 3 degrees Celsius in order to avoid the formation of moisture on the surface, which can deteriorate the quality of galvanizing.
We list the main advantages of using the cold galvanizing method to protect metal products:
- high degree of adhesion of galvanized surfaces to paints and varnishes;
- no restrictions on the size of processed products;
- low costs for preparing the product for galvanizing;
- ease of welding of cold galvanized products;
- the possibility of galvanizing at home using a sprayer, a regular paint brush or roller;
- there is no need for dismantling, transportation and reassembly of the structure being processed - all operations are performed on site.
It is also worth noting that cold galvanizing is performed in a wide temperature range (from -20 °C to +40 °C). The disadvantage of this method of galvanizing metal can be considered the low resistance of the applied coating to mechanical stress. This is an insignificant disadvantage, since the protective layer can be updated at any time.