In this article:
1. Properties of aluminum
2. Aluminum alloys
3. Raw materials for aluminum production 3.1. Aluminum ores
3.2. Bauxite
3.3. Nephelines
3.4. Alunites
3.5. Other raw materials
3.6. Cryolite production
4. Aluminum refining
Aluminum was first isolated in its free form in 1825 by the Danish physicist Oersted. Currently, aluminum is produced on an industrial scale by electrolytic means. A method for producing aluminum metal by electrolysis of alumina dissolved in cryolite was patented in 1886 independently by Paul Héroux in France and Charles Hall in the USA.
Aluminum production has developed at an extremely rapid pace since then, thanks to the importance that aluminum has acquired in industry. Until 1917, our country did not have its own aluminum smelter, although Russian scientists made a great contribution to aluminum metallurgy. In 1929, aluminum was produced at the Leningrad plant using Volkhov energy and domestic raw materials. In 1932, the Volkhov Aluminum Plant was put into operation, and in 1933, the Dnieper Aluminum Plant. Subsequently, aluminum smelters were built in various regions of our country.
The creation of a powerful energy base allowed our country to quickly become one of the first places in aluminum production.
Properties of aluminum
In its pure form, aluminum is a silvery white metal. One of the important properties of aluminum is its low density: in the solid state (at 20° C) it is 2.7 g/cm3, and in liquid form (at 900° C) it is 2.32 g/cm3. The melting point of high-purity aluminum (99.996%) is 660.24 ° C, the boiling point is 2500 ° C. Important properties of aluminum that determine its use in many areas of industry are its good electrical and thermal conductivity.
Aluminum is well machined, has good malleability, and is easily rolled into the thinnest sheet and wire. In chemical reactions, aluminum is amphoteric. It is soluble in alkalis, hydrochloric and sulfuric acids, but is resistant to concentrated nitric and organic acids. There are three valence electrons in the outer M shell of aluminum, two in the 3s orbital and one in the 3p orbital. Therefore, aluminum is usually trivalent in chemical compounds. However, in some cases, aluminum can lose one p-electron and manifest itself as monovalent, forming compounds of lower valence.
Aluminum production currently includes two main operations:
- obtaining anhydrous aluminum oxide, free from impurities accompanying aluminum, by complex chemical processing of natural compounds (bauxite, clay, kaolin);
- production of aluminum metal by electrolysis of alumina dissolved in cryolite.
Aluminum has many valuable properties: low density—about 2.7 g/cm3, high thermal conductivity—about 300 W/(m • K) and high electrical conductivity of 13.8 • 107 Ohm/m, good ductility and sufficient mechanical strength.
Aluminum forms alloys with many elements. In alloys, aluminum retains its properties. In the molten state, aluminum is fluid and fills molds well; in its solid form, it is easily deformed and can be easily cut, soldered and welded.
The affinity of aluminum for oxygen is very high. During its oxidation, a large amount of heat is released (~ 1,670,000 J/mol). Finely ground aluminum ignites when heated and burns in air. Aluminum combines with oxygen in the air and in atmospheric conditions. In this case, aluminum is covered with a thin (~ 0.0002 mm thick) dense film of aluminum oxide, protecting it from further oxidation; Therefore, aluminum is resistant to corrosion. The aluminum surface is well protected from oxidation by this film even in the molten state.
Automobile transport
One of the main requirements for materials used in automobile transport is low weight and fairly high strength indicators. The corrosion resistance and good decorative surface of the material are also taken into account.
Figure 3 – Car
The high specific strength of aluminum alloys increases the carrying capacity and reduces the operating costs of mobile vehicles. The high corrosion resistance of the material extends service life and expands the range of transported goods, including liquids and gases with high aggressive concentrations.
In the manufacture of frame elements and body trim for semi-trailers, vans, refrigerators, livestock carriers, etc. Promising materials are aluminum alloys AD31, 1915 (extruded profiles) and alloys AMg2, AMg5 (sheet).
Aluminum alloys AMts, AMgZ and 1915 are used in the manufacture of individual components of a passenger car (attachment parts, bumpers, cooling radiators, heaters).
In the US automotive industry, aluminum weldable alloys of the 3xxx, 5xxx and 6xxx series are widely used.
Beams and frames of heavy trucks are made from pressed semi-finished alloys 2014 and 6061. Panels and individual elements made from 5052 alloy are used to manufacture the cabin. As a lining material of the truck body, a sheet of alloy 5052, 6061, 2024, 3003 and 5154 is used. The body racks are made of pressed semi -finished alloys of alloys 6061 and 6063. Magnal alloys of the 5XXX series (5052, 5086, 5154 and 5454) are the main material in the manufacture of cargo stations .
Aluminum alloys
Of the aluminum alloys, duralumin and silumin are the most important.
The composition of duralumin, in addition to aluminum, includes 3.4-4% Cu, 0.5% Mn and 0.5% Mg, no more than 0.8% Fe and 0.8% Si are allowed. Duralumin deforms well and its mechanical properties are close to some types of steel, although it is 2.7 times lighter than steel (duralumin density 2.85 g/cm3).
The mechanical properties of this alloy increase after heat treatment and cold deformation. Tensile strength increases from 147-216 MPa to 353-412 MPa, and Brinell hardness from 490-588 to 880-980 MPa. In this case, the relative elongation of the alloy almost does not change and remains quite high (18–24%).
Silumins are casting alloys of aluminum and silicon. They have good casting qualities and mechanical properties.
Aluminum and alloys are widely used in many industries, including aviation, transport, metallurgy, food industry, etc. Aircraft bodies, motors, cylinder blocks, gearboxes, pumps and other parts in aviation, automotive are made from aluminum and its alloys. and tractor industry, vessels for storing chemical products. Aluminum is widely used in everyday life, the food industry, nuclear energy and electronics. Many parts of our planet's artificial satellites and spacecraft are made of aluminum and its alloys.
Due to the high chemical affinity of aluminum for oxygen, it is used in metallurgy as a deoxidizer, and also for the production of difficult-to-reduce metals (calcium, lithium, etc.) using the so-called aluminothermic process. In terms of total metal production in the world, aluminum ranks second after iron.
Development of the Russian aluminum industry
Today the Russian aluminum industry is the most developed. The Russian Federation is the second largest aluminum producer in the world. More than 3 million tons of aluminum are produced annually, most of which is exported.
Aluminum is actively used in construction due to its unique properties:
- High strength at low density;
- Resistance to natural influences;
- Durability;
- Long service life between necessary repairs.
Transport, electrical engineering, mechanical engineering, and consumer goods also require aluminum. The demand for rolled aluminum for the production of containers and packaging, sheet and profile products is growing every year. As a result, the produced aluminum is sold on the domestic and foreign markets to meet the needs of all potential and actual consumers.
However, the Russian aluminum industry also faces a number of problems
- The unfinished process of merging companies into vertically integrated groups;
- lack of own raw materials (companies are forced to import alumina); And
- low use of recycled materials in the production of domestic aluminum alloys and products made from them
- the need to transform research and development activities,
- higher level of environmental safety
To solve the above problems, it is necessary to analyze the current economic situation in the industry, assess the state of supply and sales policy, and restructure business processes.
To increase the efficiency of development of the aluminum industry, it is necessary to study the positive experience of foreign aluminum manufacturing enterprises.
Raw materials for aluminum production
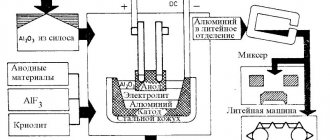
The main modern method of producing aluminum is the electrolytic method, which consists of two stages. The first is the production of alumina (Al2O3) from ore raw materials and the second is the production of liquid aluminum from alumina by electrolysis.
Aluminum ores
Due to its high chemical activity, aluminum occurs in nature only in a bound form: corundum Al2O3, gibbsite Al2O3 • 3H2O, boehmite Al2O3 • H2O, kyanite 3Al2O3 • 2SiO2, nepheline (Na, K)2O • Al2O3 • 2SiO2, kaolinite Al2O3 • 2SiO2 • 2H2O and other. The main aluminum ores currently used are bauxite, as well as nephelines and alunites.
Bauxite
Aluminum in bauxite is found mainly in the form of aluminum hydroxides (gibbsite, boehmite, etc.), corundum and kaolinite. The chemical composition of bauxite is quite complex. They often contain more than 40 chemical elements. The alumina content in them is 35-60%, silica 2-20%, Fe2O3 oxide 2-40%, titanium oxide 0.01-10%. An important characteristic of bauxites is the ratio of their Al2O3 to SiO2 content by weight - the so-called silicon module.
The silicon module of bauxite supplied to produce alumina must be no lower than 2.6. For medium-quality bauxites, this modulus is 5–7 at 46–48% Al2O3 content, and the modulus for high-quality bauxites is about 10 at 50% Al2O3 content. Bauxite with a higher Al2O3 content (52%) and modulus (10-12) is used for the production of electrocorundum.
Large bauxite deposits in our country include Tikhvinskoye (Leningrad region), Severouralskoye (Sverdlovsk region), Yuzhnouralskoye (Chelyabinsk region), Turgaiskoye and Krasnooktyabrskoye (Kustanay region).
Nephelines
Nephelines are part of nepheline syenites and urtites. A large deposit of urtites is located on the Kola Peninsula. The main components of urtite are nepheline and apatite 3Ca3(PO4)2 • CaF2. They are subjected to flotation enrichment to isolate nepheline and apatite concentrates. Apatite concentrate is used to prepare phosphate fertilizers, and nepheline concentrate is used to produce alumina. Nepheline concentrate contains, %: 20-30 Al2O3, 42-44 SiO2, 13-14 Na2O, 6-7 K2O, 3-4 Fe2O3 and 2-3 CaO.
Alunites
Alunites are the main sulfate of aluminum and potassium (or sodium) K2SO4 • Al2(SO4)3 • 4Al(OH)3. The Al2O3 content in them is low (20-22%), but they contain other valuable components: sulfuric anhydride SO3 (~ 20%) and alkali Na2O • K2O (4-5%). Thus, they, like nephelines, are complex raw materials.
Other raw materials
In the production of alumina, alkali NaOH and sometimes limestone CaCO3 are used; in the electrolysis of alumina, cryolite Na3AlF6 (3NaF•AlF3) and a little aluminum fluoride AlF3, as well as CaF2 and MgF2.
Cryolite production
Cryolite in its natural form is very rare in nature and is produced artificially from fluorspar concentrate (CaF2). The process is carried out in two stages, the first is the production of hydrofluoric acid HF. Finely ground CaF2 is mixed with sulfuric acid in tubular rotary kilns at 200 °C. The reaction takes place in the furnace: CaF2+H2SO4=2HF+CaSO4. Since fluorspar contains SiO2 as an impurity, some volatile fluorosilicic acid H2SiF6 is also formed. Gaseous HF and H2SiF6, after they are purified from impurities, are absorbed in vertical towers by water, resulting in a solution of hydrofluoric acid with fluorosilicic acid. It is purified from H2SiF6 by adding a little soda: H2SiF6 + Na2CO3 = Na2SiF + H2O + CO2. Sodium fluorosilicicate precipitates and purified hydrofluoric acid is obtained. The second stage is the production of cryolite. Al(OH)3 and soda are added to the hydrofluoric acid solution and the so-called cryolite cooking process is carried out, during which the following reactions occur:
6HF + Al(OH)3 = H3AlF6 + 3H2O
2H3AlF6 + 3Na2CO3 = 2Na3AlF6 + 3СO2 + 3H2O.
Cryolite precipitates, it is filtered and dried at a temperature of 130-150 ° C.
Aluminum fluoride is produced in a similar way by adding aluminum hydroxide to hydrofluoric acid until it is completely neutralized: 3HF + Al(OH)3 = AlF3 + 3H2O.
Aluminum refining
Aluminum extracted from electrolysis baths is called raw aluminum.
It contains metallic (Fe, Si, Cu, Zn, etc.) and non-metallic impurities, as well as gases (hydrogen, oxygen, nitrogen, carbon oxides, sulfur dioxide). Non-metallic impurities are mechanically entrained alumina particles, electrolyte, lining particles, etc. To remove mechanically entrained impurities, dissolved gases, as well as Na, Ca and Mg, aluminum is subjected to chlorination. To do this, a tube is inserted into the vacuum ladle, through which chlorine gas is supplied for 10-15 minutes, and to increase the surface of contact of the gas with the metal, porous ceramic plugs are attached to the end of the tube, ensuring the fragmentation of the gas stream into small bubbles. Chlorine reacts vigorously with aluminum to form aluminum chloride AlCl3. Aluminum chloride vapor rises through the metal layer and suspended non-metallic impurities, some gases and the resulting chlorides Na, Ca, Mg and H2 float up with them.
Next, the aluminum is poured into electric mixer furnaces or reverberatory furnaces, where it remains for 30-45 minutes. The purpose of this operation is additional purification from non-metallic and gaseous inclusions and averaging of the composition by mixing aluminum from different baths. The aluminum is then cast either into ingots on conveyor casting machines or in continuous casting plants into ingots for rolling or drawing. In this way, aluminum with a purity of at least 99.8% Al is obtained.
Higher purity aluminum is produced on an industrial scale by subsequent electrolytic refining of liquid aluminum using the so-called three-layer method. The electrolysis bath has magnesite walls, a carbon hearth (anode) and graphite cathodes suspended from above. The original aluminum is poured into the hearth through a side hole in portions, maintaining an anode layer of a certain thickness here; above it there is a layer of electrolyte made of fluoride and chloride salts, and above the electrolyte there is a layer of purified aluminum, which is lighter than the electrolyte; The ends of the cathodes are immersed in this layer.
In order for the aluminum to be refined to be at the bottom, it is made heavier, forming an aluminum-copper alloy in the anode layer (30-40% Cu is dissolved in the layer). During the electrolysis process, Al3+ ions move from the anode layer through the electrolyte layer to the cathode layer and are discharged here. The pure cathode metal that accumulates on the surface of the bath is scooped out and poured into ingots. This method produces aluminum with a purity of 99.95-99.99%. Electricity consumption is ~ 18,000 kWh per 1 ton of aluminum. Purer aluminum is obtained by zone smelting or distillation through subhalides.