Home / Food additives (E) / Preservatives (E-200 - E-299)
Back
Published: 07/25/2016
Reading time: 5 min
0
3076
Having thoroughly studied the properties of the preservative E285, Russian scientists came to the conclusion that this substance cannot be used in the food industry. It has extremely negative effects on the human body.
However, Borax is widely used in other industrial applications. Let's figure out what kind of substance this is and what properties it has?
- Substance names
- Type of substance
- Properties
- Main manufacturers
- Package
- Application
- Benefits and harms
Designation
The name BORAX is usually reserved for the name "disodium tetraborate decahydrate". However, the terms "borax anhydrous" and "borax pentahydrate" are also found in the technical and scientific literature for other forms of disodium tetraborates most widely used in industry:
- Disodium tetraborate (synonyms: boric acid , disodium salt, anhydrous borax, anhydrous borax).
- Disodium tetraborate decahydrate (synonyms: borax, borax decahydrate, tetraboric acid salt, sodium borate, boric salt, E285, borax, sodium borate, boric acid, sodium tetraborate).
- Disodium tetraborate pentahydrate (synonyms: borax pentahydrate, borax hemihydrate).
Authenticity
Qualitative reaction. To 5 ml of a 4% solution add 0.1 ml of a 0.1% phenolphthalein solution; the solution turns red. When adding 5 ml of glycerin, the 85% solution should become colorless.
- Qualitative reaction. To 0.2 g of the substance add 1 ml of concentrated sulfuric acid, 3 ml of 96% alcohol and mix. When ignited, the mixture should burn with a green-edged flame.
- Qualitative reaction. A 4% solution should give a characteristic reaction A to sodium (General Pharmacopoeia Monograph “General reactions to authenticity”).
Chemical properties
Anhydrous disodium tetraborate is stable up to 524-527 C (transition phase), melting at a temperature of about 740 C. When dissolved in water, disodium tetraborate (anhydrous, penta- or decahydrate form) is converted into boric acid and borate ions.
In dilute aqueous solutions, there are 2 types depending on the pH: if the pH is less than 7, boric acid is predominantly inseparable; if the pH is greater than 11, metaborate ions predominate, and at a pH of 7 to 11, both species coexist together. In concentrated aqueous solutions, complex ions can additionally form.
Kinds
Based on appearance, welding drills are divided into 2 types.
- Solid. In powder form, flux has the form of solid fine fractions. This shape makes it easy to lay borax on a metal surface before the soldering process, while the substance does not spread. Solid borax is sold in boxes that are sealed, thereby protecting the substance from moisture and the negative influence of the environment. In the powder fraction, borax is white.
- Divorced. This type of borax is considered the most suitable for light metal and its alloy. The substance is the same powdered borax, but dissolved in a liquid. This feature of the flux makes it possible to use it at low soldering temperatures. Using diluted borax is quite simple: small metal elements are dipped into it and then soldered. This flux is popular in jewelry, as well as when working with wires and contacts.
Applications of sodium borate
- In thermochemical processes in the production of metals;
- Glass industry: insulating glass fibers or glass wool, glass fibers for the textile industry, fibers for plastic reinforcement.
- Ceramic industry, in the production of porcelain enamels;
- In cosmetology for skin cleansing;
- As a preservative for sturgeon caviar;
- In taxidermy;
- In the production of various insecticides;
- When cleaning metals to dissolve rust;
- Production of perborates and other boron .
- In the manufacture of safety glasses resistant to high temperatures;
- In the manufacture of glasses;
- In the production of detergents, disinfectants, soaps and pesticides;
- In analytical chemistry;
- As a fertilizer for plant nutrition;
- In the making of hand forged tools such as knives and swords;
- As part of flux in the manufacture of gold products.
Description of the substance
What is borax substance? Many minerals containing sodium tetraborate have been studied. Such deposits include:
- borax or sodium tetraborate tinkal decahydrate;
- kerite;
- Borax sediment is formed during the drying up of salt lakes (Searles, lakes of Turkey);
- minerals belonging to the borate class, containing calcium, sodium and similar elements other than borax.
Varieties of borax
There are two options for this material. In solid form, borax appears as a powder with small granules of a small fraction with a white color. The flux will not spread during application and can be precisely positioned in the required metal-to-metal junction areas.
Borax powder
Borax in liquid form (sodium tetraborate)
Diluted borax has also been used for light metals. In some cases, liquids are very convenient, because you just need to dip a small part into the solution. In addition, the liquid form allows the use of borax even at low heating temperatures.
In some cases, it is advantageous to use a mixture of fluxes with borax included in the composition for the special characteristics of the base metal and special requirements for the connection.
Impact on the body
Sodium tetraborate is of low toxicity if consumed in food or if it comes into contact with the skin. However, it may cause corrosion if it comes into contact with eyes. Borax can also cause skin irritation. People who ingested sodium borate experienced nausea, vomiting, abdominal pain, and diarrhea. When inhaling borax, people complained of dry mouth, nose and throat. Some of the symptoms of overdose were cough, sore throat, shortness of breath and nosebleeds.
Children are more sensitive to the effects of borax than adults. Convulsions, confusion and coma have been reported in some children with sodium tetraborate poisoning. Sodium borate has a similar effect on animals. If ingested, the first signs of poisoning may appear within two hours.
What foods contain sodium tetraborate?
Products that contain borax can be liquid, granules, tablets, or powders. They are used in residential and commercial premises, hospitals and schools. More than five hundred products containing sodium borate are sold in the United States. Soil fertilizers, pesticides, household cleaners and detergents, laundry and personal care products.
Due to its antiseptic, antifungal, antiviral and antibacterial properties, it can be used in the treatment of cutaneous ringworm, for the disinfection of wounds and the treatment of otitis media.
What happens to sodium tetraborate when it enters the body?
Borax is rapidly absorbed when ingested with food or inhaled. It penetrates poorly upon immediate contact with the skin. But only if there is no damage to the skin. There is no evidence that sodium tetraborate breaks down naturally in the body. It can be stored in bones and adipose tissue for a long time. Most borax is excreted from the body in urine within four days of ingestion.
Has anyone studied the carcinogenic effects of long-term exposure to borax?
Studies with workers who inhaled borax over a long period of time found no negative effects of sodium tetraborate in the long term. However, they had constant complaints of vomiting, nausea, diarrhea and abdominal pain. Always follow the manufacturer's instructions on the product label!
Borax for the production of “lizuns” (smima)
Borax has a long history of use in household goods and to make slime toys, a sticky substance that is a huge hit with teenagers. This product is not safe for children due to the borax it contains, as well as solvents. You can make your own slime using cornstarch .
Borax and boric acid - what's the difference?
Borax and boric acid have different chemical compositions, so they are not the same thing, but in general both substances are dangerous because they have the same base: the chemical element boron.
It is better to replace this dangerous product with products that are much more harmless and just as effective, such as baking soda (for degreasing) or percarbonate of soda (for bleaching ).
Advantages and disadvantages of the product
Let's start with the pros:
- The drug is extremely affordable. The cost of a bottle of borax does not exceed $1, which is several times cheaper than other antiseptics and combination products.
- The drug is non-toxic, well tolerated and does not cause serious side effects.
- The product can be used in problem categories of patients: children, pregnant women, nursing mothers.
- The drug is effective, especially in gynecology. Glycerin has a softening and anti-inflammatory effect, relieving itching and burning, and boric acid helps restore the acidic protective environment of the vagina, stimulating the growth and reproduction of beneficial lactic acid bacteria.
Storage
Disodium tetraborate should be stored in special rooms accessible only to persons who have undergone special instructions. Store in tightly closed containers that are properly labeled. Try to minimize dust in the room. Provide appropriate personal protective equipment near the premises.
Disodium tetraborate can be stored in paper, cardboard, polypropylene , or polyethylene .
Disadvantages of flux
When working with borax, a characteristic deposit remains on the surface of the base metal, which must be mechanically cleaned off. Borax is susceptible to moisture and should be stored in a dry place. It is necessary to carefully prepare the flux in advance so as not to spoil the product.
Types of soldering flux
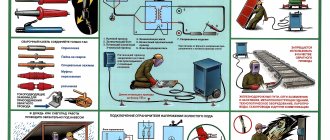
Tools and materials
The soldering technology uses a number of components.
- A soldering iron is used to heat joints that are to be soldered. Solder has a lower melting point than the metals that are being joined. Solder melts when heated with a soldering iron.
- Borax acts as a flux to prevent oxidation of the metals that are combined.
- The solder used to join copper pipes has a necessary acid base that is suitable for pipes but is corrosive to electronic connections.
- A stand on which you can hold a hot soldering iron. There are various stands. It is important to always keep the hot soldering iron in place when not in use.
- A sponge or rag that is used to clean the tip of an iron.
- Fine sandpaper used to clean connections before soldering.
- Alligator clips can be used as heat sinks if necessary.
- Burner if pipes are soldered.
Tools for soldering
Thus, the use of borax is effective in domestic conditions for cleaning surfaces and parts, and also as an antiseptic. The ingredient is often used for soldering various parts as protection against oxidation and corrosion prevention. Low cost and widespread use have allowed the substance to be used in many areas of industry and installation services.
Precautionary measures
- Instruct personnel about the risks associated with the product, the precautions to be taken and the measures to be taken in the event of an accident.
- Don't eat or drink in the workplace.
- Limit the amount of products in workshops (shops) to the minimum requirements.
- Avoid dust accumulation. Keep premises and work areas immaculately clean.
- Avoid breathing dust or aerosols.
- Provide general ventilation of the premises.
- Avoid contact of the product with skin and eyes. Provide personnel with protective clothing, gloves and safety glasses. These items must be kept in good condition and cleaned after each use.
- It is prohibited to work with tanks containing disodium tetraborate without following safety precautions.
- In case of accidental leakage or spillage, remove the product using a commercial vacuum cleaner. If the spill is large, evacuate personnel.
- Store waste in specially designed containers. Dispose of waste in accordance with approved regulations.
- Avoid exposure of disodium tetraborate to people with chronic respiratory conditions or skin conditions of exposed parts.
What is it and what is it for?
Soldering borax is a high-temperature type of powdered flux that is used when joining metal products by soldering. Melting of this substance can occur under the influence of temperatures exceeding 700 degrees Celsius. Soldering borax has its own GOST, according to which it is manufactured and its characteristics are regulated.
The substance in powder form looks very similar to salt, in other words it is called sodium tetraborate. The synthesis of borax occurs naturally, and its extraction is carried out from salt lake deposits.
The use of this substance is quite wide, but most often it is used for soldering copper pipes.
The advantages of using borax include the following:
- the materials that are planned to be processed may have different temperature conditions;
- obtaining a high-quality, reliable weld not only between metals, but also between metal and non-metal surfaces;
- ease of soldering seams if necessary to separate parts;
- when soldering, the parts do not warp or deform;
- increased productivity during capillary soldering;
- obtaining smooth and durable seams even from a craftsman with little experience.
The disadvantages of sodium tetraborate are as follows:
- release of a large volume of salts, which harden on the metal at high speed;
- absorption of moisture from the environment;
- the difficulty of selecting the right amount of borax for an inexperienced welder.
First aid
- In case of contact with skin, rinse immediately with plenty of water for 15 minutes. Remove contaminated clothing. If skin lesions or extensive or prolonged contamination are present or appear, medical supervision (clinical and biological) is required.
- In case of contact with eyes, rinse immediately and copiously with water.
- If swallowed, attempt to induce vomiting if the subject is conscious.
- If unconscious, place the victim in a safe position on his side.
- Call an ambulance!
The main thing about borax with glycerin
In appearance, borax with glycerin has a transparent 5–20% solution with a slight specific odor.
Sodium tetraborate contains:
- the main substance is borax or sodium tetraborate decahydrate (20 g)
- excipient - glycerol or glycerol (80 g).
Borax with glycerin is available in glass bottles and with a nylon and tightly screwed plastic cap, 30 g each. Borax itself acts as an antiseptic, or rather eliminates various infections, and glycerin removes irritation and helps the borax penetrate skin barriers better. Using glycerin, the solution becomes thick.
Copper pipe connection
Copper pipelines are expensive. The investment can be justified with careful installation, which is often carried out by capillary soldering using borax as a flux.
It is worth noting that today, other fluxes are sold that are more convenient to use. One pipe is inserted into the second or fitting so that the gap does not exceed 0.4 mm.
Soldering time is short, 3 minutes. It is important that the parts remain stationary during operation. In order for the borax powder to stick to the surface, the copper is first heated with a torch.
For pipes with a diameter of up to 108 mm, the soldering process is carried out at low temperatures not exceeding 450°. The seam is wide (up to 50 mm), but not very strong. Wide pipes with a diameter greater than 159 mm are soldered at high temperatures. Only professionals can perform the procedure.
In both cases, the solder melt penetrates well into the capillaries of the parts, which contributes to the formation of strong connections. It is recommended to remove any remaining borax.
It must be remembered that soldering is accompanied by the formation of smoke, so you can only work in ventilated areas.