Cyanidation, the purpose of which is to saturate the surface layers of steel with nitrogen and carbon, is one of the varieties of such a technological operation as nitrocarburization. Compared to conventional carburization, this method of steel processing is more effective, as it allows you to impart special mechanical properties to steel products.
Features of nitrocarburization and cyanidation
Although nitrocarburization and cyanidation have the same goal (saturating the surface layer of steel with nitrogen and carbon), they have one significant difference. It consists in the fact that products are subjected to nitrocarburization in a gas environment, and during cyanidation, such an environment is a melt of sodium cyanide or other salts.
Bath compositions and cyanidation modes for products
The considered technological operations demonstrate their effectiveness when processing the following materials:
- steels belonging to the stainless category;
- alloyed steel alloys, as well as steels that do not contain alloying additives and are characterized by an average carbon content in their composition;
- low carbon structural steels.
Cyanidation of steel belonging to one of the above categories, as well as the process of its nitrocarburization, occurs at a certain temperature regime (820–950°), which must be strictly observed. As a result of the qualified application of such processing methods, it is possible to solve the following problems:
- increase the wear resistance of the product surface;
- increase its surface hardness;
- increase the endurance limit of the metal.
Nitrocarburization in various environments
There is another type of carburization called soft nitriding. This treatment, which is performed at a temperature of about 590°, is needed for medium-carbon steels to increase their level of wear resistance and endurance limit. Products made from high-speed steels are also subjected to cyanidation, which increases the hardness and wear resistance of their surface layer, as well as making it more resistant to elevated temperatures.
The metallurgical industry also uses a technological operation such as cyanidation of gold ores, which is fundamentally different from all of the above steel processing methods. The purpose of cyanidation of ore, which may contain gold even in very small quantities, is to extract from it a concentrate characterized by a high content of precious metal. Such a concentrate, after further processing, can be used for the production of gold products.
Gold mining using cyanidation method
This is interesting: All about metal carburization using the example of steel
Advantages of the methods
Nitriding and nitrocarburization are several times more effective than the cementation process. The cemented layer with a martensitic structure retains its improved properties provided that the temperature does not exceed 225°C. The nitriding technique allows you to increase the indicators to 550 - 600°C. This method is most effective for increasing the wear resistance of austenitic stainless steels. Nitrocarburization combines the advantages of carburization and nitriding; this process is more progressive and economical: the saturation of the metal surface with nitrogen and carbon is accelerated by 30 - 35%, the production cycle is reduced by 50 - 60%, deformations are minimal, the wear resistance of the metal and the ability to harden are increased.
Definition of the word “cyanidation” according to TSB:
Cyanidation - in hydrometallurgy, a method of extracting metals (mainly gold and silver) from ores and concentrates by selectively dissolving them in solutions of alkali metal cyanides. Selectivity of dissolution is achieved by the low concentration of the solution (0.03-0.3% cyanide), due to which it has little interaction with other components of the ore. The dissolution of gold and silver in a cyanide solution occurs in the presence of oxygen dissolved in water. increasing its concentration intensifies the process (see Cyanide). To prevent the decomposition of cyanides, a protective alkali in the form of lime or caustic soda is introduced into solutions in an amount of 0.005-0.02%. The theory of cyanide processes is based on the laws of the kinetics of dissolution on a non-uniform surface (with cathodic depolarization by oxygen) and the diffusion dissolution of metals (with simultaneous diffusion of cyanide and oxygen). Of great importance are the patterns of interaction of reagents with minerals, taking into account their composition and structure. In industry, two methods of coloring are used: percolation (percolation) of solutions through a layer of finely crushed ore or sand and mixing of the pulp with intense aeration. From solution, gold and silver are often precipitated by zinc dust. Sorption coloring is being developed, combining the processes of leaching and extraction of dissolved gold and silver from pulp by sorption with anion exchangers or activated carbons. This type of carbon is effective in processing difficult-to-filter slurry ores. Gold recovery from pulp coloring is 90-96%, with a consumption of sodium cyanide of 0.25-3 kg/t and protective alkali of 0.5-5 kg/t. The dissolution of gold and silver in cyanide solutions was first studied in 1843 by P. R. Bagration. His research was supplemented by F. Elsner (Germany, 1846) and M. Faraday (1856). C. entered production practice in the early 90s. 19th century (patents of J. MacArthur and brothers R. and W. Forrest, Great Britain, 1887 and 1888). See also Noble metals, Hydrometallurgy. Lit.: Maslenitsky I.N., Chugaev L.V., Metallurgy of noble metals, M., 1972. Fundamentals of metallurgy, vol. 5, M., 1968. Cyanidation - steel, a type of chemical-thermal treatment, consisting in complex diffusion saturation of the surface layer of steel with carbon and nitrogen in melts containing cyanide salts at 820-860°C (medium temperature temperature) or at 930-950°C (high temperature temperature). The main purpose of steel is to increase the hardness, wear resistance, and endurance limit of steel products. In the process of fermentation, cyanide salts are oxidized, releasing atomic carbon and nitrogen, which diffuse into the steel. At medium temperature, a cyanidated layer 0.15-0.6 mm deep with 0.6-0.7% C and 0.8-1.2% N is formed; at high temperature (this type of carbonation is often used instead of carburization) - a layer 0.5-2 mm deep with 0.8-1.2% C and 0.2-0.3% N. After hardening, the product is subjected to hardening and low tempering. Disadvantages of cyanide: high cost, toxicity of cyanide salts and the need in this regard to take special measures to protect labor and the environment. C. differs from nitrocarburization, in which saturation with nitrogen and carbon is carried out from a gaseous medium. Lit.: Minkevich A.N., Chemical-thermal treatment of metals and alloys, 2nd ed., M., 1965. Lakhtin Yu.M., Metallurgy and heat treatment of metals, 2nd ed., M., 1977. Yu. M. Lakhtin.
Gas siliconization
In the process of this type of carburization, siliconization, the top layer of steel is saturated with silicon, which makes the part resistant to acids, wear-resistant, and heat-resistant. Siliconization can be performed in one of three cementators.
Solid siliconization. It is customary to use ferrosilicon and fireclay as a medium. To reduce the amount of time, aluminum chloride can be added. The temperatures of such cementation are quite high - up to 1200 °C. If the part is kept for 10 hours, the layer thickness will be 0.7 millimeters.
Liquid siliconization. For this type of cementation, chloride salt is used, to which ferrosilicon is added. Holding temperature – 1000 °C.
Gas siliconization
Gas siliconization. It has the most important meaning in industry. The process is very intensive. The holding temperature can reach 1050 °C, the time can be from 2 to 6 hours, the layer thickness can be up to 1 millimeter.
An important feature of the surface layer, which is saturated with silicon, is its porous structure. Oil can change the situation a little; to do this, the part must be boiled in it at a temperature of 200 °C. The resulting material will be quite heat-resistant and durable.
Advantages and disadvantages of nitrocarburization
Among the advantages of nitrocarburization, one can note the high technological effectiveness of the process, simplicity and convenience of adjusting parameters. By selecting the temperature regime, the composition of the gas mixture and, in particular, the processing time, you can easily adjust the thickness of the saturated layer depending on the requirements. The low processing temperature reduces the risk of product deformation and simplifies further hardening, since only minimal time is needed to reduce the temperature of the workpiece. Thus, the technological cycle time for production of products is reduced. The processed products have high surface quality and excellent physical and mechanical properties. In low-alloy steels, after treatment, an increase in corrosion resistance is observed.
Microstructure of nitro-carburized layers
Among the many useful properties, we must not forget that this metal processing technique also has disadvantages. The most significant disadvantage of this type of nitrocarburization, such as cyanidation, is the high toxicity of the production components. To saturate with nitrogen and carbon, sodium and calcium cyanide salts, which are extremely toxic substances, are used.
A less significant drawback, which is insignificant in many areas of application, is the slightly increased fragility of the metal after processing. But since the changes affect only a relatively thin layer, this characteristic is insignificant and is offset by the increased resistance of the material to wear.
When producing parts that require cyanidation and subsequent hardening, it is necessary to strictly observe the sequence and timing of the parts of the technological process. Thus, hardening should be carried out immediately after the end of the saturation process, since reheating the workpiece will lead to the outflow of nitrogen molecules from the treated surface. The reduction in nitrogen concentration can be up to 60%.
As already mentioned, low processing temperatures make it possible to combine several types of processing in a single process. After completion of the saturation process, the parts require a short cooling time for further quenching in oil. Thus, oil hardening can be done directly in a nitrocarburization furnace.
All types of nitrocarburization, by accelerating the saturation of steel with carbon compared to carburization, provide an advantage in processing time of up to 50-60%. Thus, the main benefits of nitrocarburization are reduced production time with minimal risk of negative impact on part geometry. At the same time, performance is improved due to the presence of nitrogen.
The composition of the gas mixture is quite simple to regulate both before and during processing. The heating time of the process components is significantly reduced, since the gas supplied to the chamber may already have the required temperature.
Since the processes of nitrocarburization and carburization are technologically very similar, the same equipment can be used for them, which greatly facilitates the transition to a different range of products or changes in production technology.
What is metal heat treatment called: basics, general principles
In the process of this technology, the crystal lattice of the blank is transformed. The task is to change the properties, not its configuration and dimensions. Upon completion, the workpiece acquires the parameters required by the technology and a unique structure. Let's look at why metal hardening is needed and how it affects the structure of steel after the procedure:
- • to improve technological characteristics through softening, this process is used as a preparatory operation or an intermediate stage;
- • to obtain the required technical characteristics by strengthening or acquiring a specialized structure;
- • to fix the size and configuration, as well as obtain new properties of the workpiece.
How to prepare a part
The surfaces of the part must be cleaned and degreased before nitrocarburizing. To do this, it is enough to wash them for 15 minutes in a solution of caustic soda heated to 90 ° C, or you can wipe them with gasoline. Then the parts are wiped dry and placed in baskets at a distance sufficient for free penetration of gas.
What can be saturated with carbon?
It is advisable to carry out nitrocarburization with stainless steel, alloys containing alloying additives, and structural steels with low carbon content.
Properties and application of cyanidated steels
Steels that have undergone cyanidation treatment differ sharply from conventional steels by increasing the fatigue strength parameter, the endurance limit. The scope of application of such steels is varied:
- welded construction structures;
- lantern and window frames in industrial buildings;
- various small hardware: washers, pawls, rivets, sprockets, couplings - everything that is used at temperatures down to -40 degrees Celsius;
- gears, shafts in mechanisms where friction is present.
This is interesting: Features of hardening of various types of steel - methods, temperature, other nuances
Statement
The ore is crushed using grinding equipment. Depending on the ore, it is sometimes further concentrated using froth flotation or. Water is added to make a paste
or
pulp
. The base ore slurry may be combined with a solution of sodium cyanide or potassium cyanide; many operations use calcium cyanide, which is more economical.
To prevent the creation of toxic hydrogen cyanide during processing, slaked lime (calcium hydroxide) or soda (sodium hydroxide) is added to the extractant solution to ensure that the acidity during cyanidation is maintained over a pH of 10.5 – highly alkaline. Lead nitrate can improve gold leaching rates and quantities, especially when processing partially oxidized ores.
Effect of dissolved oxygen
Oxygen is one of the reactants consumed during cyanidation by accepting electrons from the gold, and a lack of dissolved oxygen reduces the rate of leaching. Air or pure oxygen gas can be blown through the pulp to maximize dissolved oxygen concentration. Internal oxygen-pulp contactors are used to increase the partial pressure of oxygen contacting the solution, thus raising the dissolved oxygen concentration well above the saturation level at atmospheric pressure. Oxygen can also be added by dosing the pulp with a solution of hydrogen peroxide.
Pre-aeration and ore washing
In some ores, especially partially sulfided ones, aerating (before introducing cyanide) the ore into water at high pH can make elements such as iron and sulfur less reactive to cyanide, making the gold cyanidation process more efficient. In particular, the oxidation of iron to iron(III) oxide and subsequent precipitation as iron hydroxide minimizes the loss of cyanide due to the formation of ferrous cyanide complexes. Oxidation of sulfur compounds to sulfate ions avoids the conversion of cyanide to the byproduct thiocyanate (SCN – ).
Technology, purpose and types of nitrocarburization
Nitrocarburization is the saturation of a steel product with carbon and nitrogen, which occurs in an environment of cementing gas with the addition of dissociated ammonia. By changing the composition of the gas and the temperature at which the nitrocarburization process occurs, you can influence the percentage of carbon and nitrogen in the resulting layer. The layer thickness can also be controlled by choosing the temperature and holding time. The process of nitrocarburization is diffusion. There are high-temperature and low-temperature nitrocarburization of steel. The first method of nitrocarburization is used at temperatures ranging from 830 to 950 degrees Celsius. In this case, ammonia is used in higher doses. After the operation, hardening measures with low tempering are carried out on the product. The hardness achieved as a result corresponds to 62–56 HRC. Steels that are mainly subjected to nitrocarburization are carbon (they are used to make mechanical engineering parts) and low-alloy metals.
The low-temperature nitrocarburization method involves the use of a thermal environment in the range of 530–570 degrees for a duration of no more than 3 and no less than 1.5 hours; the parts are first hardened and tempered. The resulting hard layer has a thickness of 0.004 to 0.02 millimeters with a strength of 1200–900 HV.
Nitrocarburization is a safe process with low operating costs, which is why it is often used in the automotive industry.
Anti-corrosion nitriding
To increase the corrosion resistance of steel parts on the surface, it is necessary to obtain a non-porous, non-etching, anti-corrosion phase layer (0.01-0.03 mm), which is resistant to atmosphere, gasoline, and weak alkaline solutions. Nitriding in this case is carried out at t = 600-700°C, the process duration is 0.5-1.5 hours. It also increases hardness, tensile strength and endurance. However, this does not impose high demands on mechanical properties, so anti-corrosion nitriding is carried out at a higher temperature and on any steel, even ordinary carbon steel. Depending on the process conditions, gas and liquid nitriding are distinguished. Nitriding in liquid media is called the tenifer process and is carried out in melts of 40% KNC + 60% NaCN, t = 550-570°C, t = 0.5-0.3 hours. When blowing dry air, a 7-15 µm layer of Fe3(CN) carbonitrides with high wear resistance is formed on the surface. The advantage of the method is a slight change in size, and the disadvantage is the toxicity and high cost of cyanide salts. Quality control of nitriding is carried out by hardness, layer depth on witness samples, and external inspection of the nitriding surface.
Saturation of the steel surface simultaneously with carbon and nitrogen is called cyanidation. Cyanidation is used to increase the surface hardness, wear resistance and installation strength of steel parts. Cyanidation can be carried out in solid, liquid and gaseous media, therefore liquid and solid cyanidation are distinguished; gas cyanidation is called nitrocarburization. Nitrogen, penetrating into steel simultaneously with carbon, lowers the critical Asz and promotes intensive carburization of steel at a lower temperature than during carburization. In addition, nitrogen accelerates the diffusion of carbon in austenite. Therefore, cyanidation has the following advantages:
1. The productivity of the planning process is slightly higher than cementation due to the greater speed of the process;
2. A lower cyanidation temperature of 840-860° C helps to reduce the deformation of parts, increase the durability of furnace equipment, and makes hardening possible immediately after cyanidation;
3. The diffusion layer obtained as a result of cyanidation or nitrocarburization has special properties that differ from the properties of only cemented or nitrided layers.
For planning, steels containing 0.2-0.4% C, carbon or low-alloy steels, tool steels, and high-alloy steels are often used, which are subjected to planning to increase wear resistance. The most common are liquid planning and nitrocarburizing.
Liquid cyanidation is carried out in molten salts:
cyanide (NaCN, KCN, K4 and neutral (NaCl, BaCl, NaC03, KCl and others). Recently, a bath consisting of 20-25% NaCN, 25-50% Na2CO3 has been more widely used. Oxygen dissolved in a liquid bath, interacts with NaCN
4NaCN+02 >4NaCNO
4NaCNO + 02 > 2Na2C03 + 2СО + 4N (atom)
2CO>CO2+C(atom).
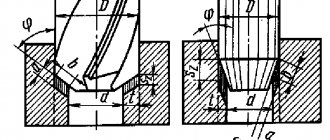
Atomic N and C diffuse into iron, and as a result of the reactions Na2C03 remains. Cyanidation is carried out at t = 820-870°C, and then the parts are subjected to hardening at the cyanidation temperature and low tempering (180-200 °C) (Fig. 82). The microstructure consists of a thin layer of carbonitrides Fe2 (N,C); Fe3 (C,N) and nitrogenous martensite. To obtain a layer of greater depth, increase the temperature of cyanidation (deep cyanidation) to t = 900-950 ° C in a bath containing 8% NaCN, 82% BaCl, 10% NaCI
BaCl2 + 2NaCN →NaCI + Ba(CN)2
Ba(CN)2 → Ba(CN)2+C(atom).
Ba(CN) 2 +02 →BaO+CO+2N (atom).
After high-temperature cyanide plating, the parts are cooled in air, and then heated again for hardening to refine the grains.
The higher the cyanidation temperature, the closer this process is to carburization. Deep cyanidation is used instead of cementation, because it requires less time to obtain a layer of a given thickness, significantly less deformation of parts and higher resistance to wear and corrosion.
Tools made from high-speed and high-chromium steels are subjected to low-temperature cyanidation at t = 550-600° with the steel surface being saturated with nitrogen. The disadvantage of liquid cyanidation is the toxicity and high cost of cyanide salts.
Nitrocarburization is carried out in a gas environment consisting of carburizing gas and dissociated ammonia at t=850-860°C. Depending on the ammonia and process temperature, the concentrations of nitrogen and carbon in the surface layer of steel are different. Thus, at t = 850-870°C in an atmosphere with a minimum amount of NНз (3%), alloy steels are saturated with carbon much more intensely than with nitrogen. This process is proposed to be called carbonitridation. Carbon and low-alloy steels are subjected to nitrocarburization at 850-870°C and a high NH3 content. There are high and low temperature nitrocarburization. After high-temperature nitrocarburization at t = 840-860°C, hardening is carried out directly either from the nitrocarburization temperature or after cooling to 800-820°C and low tempering. Quality control of heat treatment is carried out on witness samples made of the same grade of steel as the parts being processed, which have undergone chemical-thermal treatment along with the parts. The thickness of the layer and its structure are determined metallographically. After quenching and tempering, the hardness is measured on the surface of the parts, which should be within HRC 58-64. The structure of the surface layer after nitrocarburization and hardening consists of fine-crystalline martensite, 25-30% carbonitrides and retained austenite. Moreover, the amount of retained austenite in the nitrocarburized layer is greater than in the cemented layer due to alloying of the former with nitrogen. The increased amount of retained austenite in the nitrocarburized layer explains the increased ductility, toughness and good running-in properties of parts after nitrocarburization.
Nitrocarburization is a cheaper process than liquid cyanidation, safe, and allows more precise control of the thickness and composition of the diffusion layer.
Compared to gas carburization, nitrocarburization is carried out at a lower temperature; but at the same speed, no soot is released on the surface of the parts, the diffusion layer has higher wear resistance, endurance limit, with nitrocarburization there is less deformation of parts, and the cost of the process is lower. Therefore, nitrocarburization is quickly being introduced into industry instead of gas carburization. For nitrocarburization in shaft furnaces, a liquid cyanogen triethanolamine (C2H4OH)3N is used, which is introduced into the working space in the form of drops. At temperatures of 550-950°C it decomposes according to the reaction:
M(C2H40H)3 -2CH4 +HCN+3CO+3H2
CH4, HCN and CO provide the production of atomic C and N. In recent years, low-temperature nitrocarburization has begun to be used at t = 570-600°C in an atmosphere of carbon-containing gases and ammonia, which can replace liquid nitriding in molten poisonous cyanide salts.
Steel Cementation
Cementation of steel - chemical-thermal treatment of low-carbon steel by surface saturation (from Table 1
Temperature, °С Heat colors Temperature, °С Heat colors
1600 Dazzling white blue 850 Light red 1400 Bright white 800 Light cherry 1200 Yellow white 750 Cherry red 1100 Light white 600 Medium cherry 1000 Lemon yellow 550 Dark cherry 950 Bright red 500 Dark red 900 Red 400 Very dark red (visible in the dark)
A thin film of iron oxides that gives the metal a variety of rapidly changing colors, from light yellow to gray. Such a film appears if a steel product cleaned of scale is heated to 220°C; As the heating time increases or the temperature rises, the oxide film thickens and its color changes. Tarnish colors appear equally on both raw and hardened steel.
With low tempering (heating to a temperature of 200-300°), martensite mainly remains in the steel structure, which, however, changes the lattice. In addition, the separation of iron carbides from the solid solution of carbon in alpha iron begins and their initial accumulation in small groups. This entails a slight decrease in hardness and an increase in the plastic and viscous properties of steel, as well as a decrease in internal stresses in parts.
For low tempering, parts are kept for a certain time, usually in oil or salt baths. If for low tempering parts are heated in air, then tarnish colors appearing on the surface of the part are often used to control the temperature.
| Tarnish color | Temperature, °C | A tool to be released |
| Pale yellow | 210 | — |
| Light yellow | 220 | Turning and planing tools for machining cast iron and steel |
| Yellow | 230 | Same |
| Dark yellow | 240 | Coins for coining by casting |
| Brown | 255 | — |
| Brown-red | 265 | Dies, drills, cutters for processing copper, brass, bronze |
| Violet | 285 | Chisels for steel processing |
| Dark blue | 300 | Coining coins for sheet copper, brass and silver |
| Light blue | 325 | — |
| Grey | 330 | — |
The appearance of these colors is associated with the interference of white light in iron oxide films that appear on the surface of the part when it is heated. In the temperature range from 220 to 330 °, depending on the thickness of the film, the color changes from light yellow to gray. Low tempering is used for cutting, measuring tools and gears.
With medium (heating in the range of 300-500°) and high (500-700°) tempering, steel goes from the martensite state to the trostite or sorbite state, respectively. The higher the tempering, the lower the hardness of the tempered steel and the greater its ductility and toughness.
With high tempering, steel receives the best combination of mechanical properties, increasing strength, ductility and toughness, therefore high tempering of steel after hardening to martensite is prescribed for forging dies, springs, springs, and high tempering for many parts exposed to high stresses (for example, axles cars, engine connecting rods).
For some steel grades, tempering is carried out after normalization. This refers to fine-grained alloy hypoeutectoid steel (especially nickel), which has high toughness and therefore poor machinability with cutting tools.
To improve machinability, steel is normalized at elevated temperatures (up to 950-970°), as a result of which it acquires a large structure (determining better machinability) and at the same time increased hardness (due to the low critical hardening rate of nickel steel). In order to reduce hardness, high tempering of this steel is carried out.
Main defects during nitrocarburization
During the nitrocarburizing process, defects in the processed parts may occur.
Peeling
This phenomenon occurs when the surface of the part is saturated with carbon and is associated with too low temperatures or rapid heating. In the first case, the carbon content levels out too slowly towards the center. With rapid heating, the carbon content decreases sharply with distance from the surface of the part. Such sudden changes provoke separation of the cemented layer from the product in the form of peeling of the shell.
Coarse-grained fracture
The coarseness of the treated layer can be caused by several factors: overheating, overexposure during hardening, excess carbon in the cemented layer due to high or changing temperatures during processing. These defects can be eliminated by re-hardening. Coarse graining of the core can occur due to the quenching temperature being too low. And if we are talking about low-alloy or carbon steels, then this defect can be explained by the parts being too large, which does not allow the core to be sufficiently calcined.
Soft surface
This defect in the surface of processed products is caused by a number of violations of the nitrocarburization process (the appearance of voids when stuffing parts, the appearance of a graphite crust on the surface of the part). Such a defect can also be caused by a hardening defect associated with a low cooling rate or the formation of a steam jacket. When nitriding, soft spots are associated with the processing of non-greased parts.
Low saturated film thickness
This defect occurs at low nitriding temperatures. The flaw is extremely dangerous, since it is impossible to detect it using conventional control methods. But the problem can be eliminated by repeating the procedure while observing the temperature regime.
Purpose of the process
Normalization is designed to change the microstructure of steel; it does the following:
- reduces internal stress;
- through recrystallization, it refines the coarse-grained structure of welds, castings or forgings.
The goals of normalization can be completely different. Using this process, the hardness of steel can be increased or decreased, the same applies to the strength of the material and its toughness. It all depends on the mechanical and thermal characteristics of the steel. Using this technology, it is possible to both reduce residual stresses and improve the degree of machinability of steel using one or another method.
Steel castings are subjected to this treatment for the following purposes:
- to homogenize their structure;
- to increase susceptibility to heat hardening;
- to reduce residual stresses.
Products obtained by forming are subjected to normalization after forging and rolling in order to reduce the heterogeneity of the structure and its banding.
Normalization together with tempering is needed to replace the hardening of products with complex shapes or with sharp changes in cross-section. This will prevent defects.
This technology is also used to improve the structure of the product before hardening, increase its machinability through cutting, eliminate the secondary cement network in hypereutectoid steel, and also prepare the steel for final heat treatment.