Difference between Low Alloy Steel and High Alloy Steel
The main difference between Low Alloy Steel and High Alloy Steel is that Low Alloy Steels contain less than 0.25% alloying element whereas High Alloy Steels have more than 10% alloying element .
In addition to dividing into low-alloy and high-alloy steel, it is also divided according to the degree of alloying into medium-alloy. In this steel, the amount of alloying elements ranges from 2.5 to 10%). The alloy is a mixture of two or more elements. It is produced by mixing molten metal with some other elements (metals or non-metals or both) to produce a material that has improved properties over the original metal. Low alloy steel and high alloy steel are two types of iron alloys with alloying elements. The most popular alloying elements in these steels are: nickel (Ni), copper (Cu), titanium (Ti) and vanadium (V), nitrogen (N), etc.
Content
- Overview and main differences
- What is Low Alloy Steel
- What is High Alloy Steel
- What is the difference between Low Alloy Steel and High Alloy Steel
- Conclusion
What is Low Alloy Steel?
Low alloy steel is a type of alloy steel whose properties are improved compared to carbon steel. For example, this alloy has better mechanical properties and greater corrosion resistance than carbon steel. The carbon content of low alloy steel is less than 0.2%. The most common alloying elements in this steel are: Nickel (Ni), Chromium (Cr), Molybdenum (Mo), Tungsten (V), Boron (B), Tungsten (W) and Copper (Cu).
In most cases, the manufacturing process of these alloy steels includes heat treatment and tempering (for normalization). But now, there is a tendency to harden and temper. In addition, almost all low alloy steel materials are weldable. However, the material sometimes requires treatment before or after welding (to avoid cracking).
Some advantages of low alloy steel:
- Yield strength is higher
- High tensile strength
- Higher resistance to oxidation and corrosion
- Low cold brittleness threshold
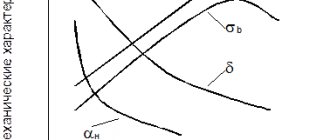
This material is used in industry, but up to a maximum temperature of 580 °C. If the temperature is higher than 580 °C, this material is not suitable due to lack of sufficient oxidation resistance to cope with high temperatures.
What is High Alloy Steel?
High alloy steel is a type of alloy steel that contains more than 10% alloying elements. Unlike low alloy steel, the alloying elements for high alloy steel are chromium (Cr) and nickel (Ni). The most famous example of this steel is stainless steel.
Chromium provides steel with a thin oxide layer on the surface of the steel. This is called the hidden layer because this layer retards corrosion of the metal. In addition, manufacturers typically add large amounts of carbon and manganese to give the steel its austenitic character. In addition, this material is more expensive than low alloy steel.
What is the difference between Low Alloy Steel and High Alloy Steel?
Both low-alloy and high-alloy steel have improved properties than carbon steel. However, the key difference between low alloy steel and high alloy steel is that low alloy steels contain less than 0.25% alloying elements, while high alloy steels contain more than 10% alloying elements. In the chemical composition, low alloy steel contains iron, carbon (less than 0.2%) and other alloying elements such as Nickel (Ni), Chromium (Cr), Molybdenum (Mo), Tungsten (V), Boron (B), Tungsten ( W) and Copper (Cu), while high alloy steel contains iron, chromium, nickel, carbon, manganese, etc.
Conclusion – Low Alloy Steel vs High Alloy Steel
Both low-alloy and high-alloy steel have improved properties than carbon steel. The main difference between Low Alloy Steel and High Alloy Steel is that Low Alloy Steels contain less than 0.25% alloying elements whereas High Alloy Steels have more than 10% alloying elements.
Source of the article: https://raznisa.ru/raznica-mezhdu-nizkolegirovannoj-stalju-i-vysokolegirovannoj-stalju/
Heat-resistant steels
Heat-resistant steels - the main distinguishing features are high long-term strength and creep. Steel, while withstanding loads, does not deform or collapse. They are used in the production of equipment and structures that operate for a long time under the influence of high temperatures - turbines, rotors for power plants, equipment for boiler houses, furnaces with high temperatures, all kinds of engine valves, in the construction of especially critical structures in the open air, where metal corrosion cannot be allowed. Such unique properties are obtained by adding chromium to the alloy.
Content:
The key difference between low alloy steel and high alloy steel is that low alloy steels contain less than 0.2% alloying element, whereas high alloy steels contain more than 5% alloying element. .
An alloy is a mixture of two or more elements. It is produced by mixing a metal with some other elements (metals, non-metals or both) to produce a material that has improved properties compared to the original metal. Low alloy steel and high alloy steel are two types of iron alloys.
Quality classification
Alloyed and unalloyed steel within each grade differs in quality, which depends on the production technology and the quality of the starting materials.
The quality of steel is particularly affected by impurities that remain in it during the reduction of iron from ore concentrates. Phosphorus and sulfur mainly negatively affect the quality of steel. According to their content, steel of ordinary quality and high-quality steel are classified, at the end of which there is the letter A. The phosphorus content in high-quality steel does not exceed 0.025%.
What is low alloy steel?
Low alloy steel is a type of alloy steel whose properties are improved compared to carbon steel. For example, this alloy has better mechanical properties and greater corrosion resistance than carbon steel. The carbon content in low alloy steel is less than 0.2%. Alloying elements other than carbon include Ni, Cr, Mo, V, B, W and Cu.
In most cases, the production process of this alloy steel involves heat treatment and tempering (for normalization). But now it usually involves hardening and tempering. In addition, almost all low alloy steel materials are weldable. However, the material sometimes requires treatment before or after welding (to prevent cracking).
Some benefits of low alloy steel include the following:
- Yield strength
- Creep force
- Oxidation resistance
- Hydrogen resistance
- Low temperature plasticity, etc.
Additionally, this material is very useful in industry, but at temperatures below 580°C. If the temperature is higher, this material is no longer suitable due to insufficient oxidation resistance to withstand high temperatures.
Case-hardened steels
Case-hardening steels are steels in which the surface layer, under the influence of high temperature, is enriched with carbon atoms. At the same time, while obtaining a durable, anti-corrosion surface, the inner layer of the alloy remains viscous and ductile. Such steel is used for the production of shafts, gears, and small minor spare parts. If nickel, manganese, and titanium are added, a stronger steel is obtained and more complex structures and parts are made from it that can withstand sharply changing, long-term mechanical stress.
What is high alloy steel?
High alloy steel is a type of alloy steel in which the alloy steel content is more than 5%. Unlike low-alloy steel, the alloying elements for high-alloy steel are chromium and nickel. One well-known example of this type of material is stainless steel.
Chromium provides steel with a thin oxide layer on the surface of the steel. We call this the latent layer because it retards corrosion of the metal. Moreover, manufacturers usually add large amounts of carbon and manganese to give the steel its austenitic character. In addition, this material is more expensive than low-alloy steel.
Comparison of carbon steel with stainless steel
Ability to absorb odors
Carbon steel itself has a pleasant smell (especially when clean and freshly sharpened), but it quickly absorbs foreign odors. If we plan wood with a carbon knife, this is even great, but for cutting food it is not very good: the “aroma” of onions or smoked fish will take a long time to wash off the tool. There are no such problems with stainless steel; it itself is also odorless. For kitchen knives this is a definite plus.
What is the difference between low alloy steel and high alloy steel?
Both low alloy and high alloy steel have improved properties than carbon steel. However, the key difference between low alloy steel and high alloy steel is that low alloy steels contain less than 0.2% alloying element, while high alloy steels contain more than 5% alloying element. When considering the chemical composition, low alloy steel contains iron, carbon (less than 0.2%) and other alloying elements such as Ni, Cr, Mo, V, B, W and Cu, while high alloy steel contains iron, chromium, nickel, carbon, manganese, etc.
The infographic below provides further information on the difference between low alloy steel and high alloy steel.
Description of the main grades of high-alloy steel
The purpose of adding alloying components is to change the physical properties of steel - increasing strength, resisting corrosion, increasing flexibility. Depending on the concentration of alloying elements, three types are distinguished: low-alloy steel (additional components less than 2.5%), medium-alloy steel (from 2.5 to 10%) and high-alloy steel (from 10 to 50%).
What are the main differences between high-alloy steel? What grades of high-alloy steels exist? And what should you remember when carrying out welding work? Below we will find out the answers to these questions.
Tool steels
Tool steels have sufficient strength, hardness and wear resistance, and are used for the manufacture of high-precision equipment, measuring instruments and various tools. This steel must be easy to process, grind, and be resistant to overheating, calcination, and cracking. Having low toughness and significant brittleness, steel does not tolerate sharp impacts and the material can collapse.
This subgroup includes high-speed steel, which has a high hardness coefficient, red-hardness (up to 600°C), and high resistance to fracture. It allows you to produce tools that cut at high speeds without causing damage to the cutting equipment. Such unique properties are created by a number of specially selected alloying components introduced into the alloy.
Key Features
High-alloy steel, in addition to carbon and iron, contains a large amount of alloying additives (from 10 to 50%). As additional components: chromium, nickel, silicon, manganese, tungsten, molybdenum, vanadium, aluminum, cobalt, titanium, as well as various rare earth metals.
Most often, chromium and nickel act as additional components - the remaining components are usually contained in small quantities. Although there are some exceptions: a simple example - austenitic grades of high-alloy steels can contain manganese in a concentration of 1 to 15%.
The reasons for adding alloying additives are very simple - they change the structure and physical properties of the steel alloy, which allows a person to obtain a metal with the desired properties.
Categories
Categories of high-alloy steels depending on their physical properties:
- Scale-resistant (heat-resistant) high-alloy steels. The main feature of such alloys is complete resistance to moderate to high temperatures (up to 550 degrees Celsius) of the environment in an unloaded state. In other words, such steels can withstand overheating well for a long time if they do not need to support any heavy weight. Please note that in addition to high temperatures, scale-resistant steels also tolerate long-term exposure to moderately toxic chemicals.
- Heat-resistant high-alloy steels. Based on the name, you might think that heat-resistant and heat-resistant alloys are the same thing, but this is not entirely true. Heat-resistant alloys can withstand high temperatures (up to 800 degrees and above) under high load conditions, but for a short time. In other words, such alloys can withstand high heat for a short period of time (whereas heat-resistant alloys can withstand moderate heat for a long time). Short-term stability also applies to highly toxic chemicals.
- Anti-corrosion (stainless) steel alloys. They are fully resistant to all main types of corrosion (surface, crystalline, electrochemical, and so on). Please note that in the composition of such alloys, the alloying components are evenly distributed throughout the steel alloy, which makes the material evenly resistant to all anti-corrosion influences. Why is this so important? A simple example: when chrome plating, only an external anti-corrosion coating is formed, which can be damaged or erased for natural reasons - high-alloy alloys contain anti-corrosion additives throughout the metal, which makes such alloys more stable.
Relevance
The high wear resistance and heat resistance of rolled stainless steel, immunity to aggressive environments, allows it to be used where work involves contact with hot aggressive environments. Unique performance characteristics make it possible to use it for the production of heat exchange equipment, heat shields, parts for thermal furnaces, and combustion chambers. Corrosion resistance makes it relevant in industrial chemistry, for welded chemical equipment. The aesthetic appeal of such a sheet is important in construction and design.
Alloy steel marking
According to GOST, for marking alloy steel (low-, medium- and high-alloy) special codes are used that display the approximate composition of a particular grade. In a technical sense, ciphers have the form of an alphanumeric sequence, which has the following structure: XXXYYYZZZ (all characters are written together and without deviations). The decoding of the code is as follows:
- XXX is a special letter prefix that indicates the type of steel alloy (we will give the decoding below).
- YYY - This fragment is a number that represents the amount of carbon in the alloy. If there are two numbers, this means that the carbon content is expressed in hundredths of a percent. If there is one number, the carbon content is in tenths of a percent.
- ZZZ is an alphanumeric sequence that displays alloying components and their approximate quantity (we will also give a decoding below).
Decoding
Let's now look at the XXX prefix - this code indicates the special properties of steel. In a technical sense, it represents one or more letters (most often one) that denotes a particular property. The XXX prefix has almost fallen into disuse and is rarely used in practice. The main values that the prefix can take are presented in the table below:
| Prefix character XXX | Prefix decoding |
| E | Electrical steel |
| A | Automatic steel |
| R | Cutting steel |
| L | Cast steel |
The ZZZ sequence indicates the presence of additional alloying components in the steel alloy. If any component in a steel alloy is contained in a concentration of more than 1%, then the percentage of this element is indicated next to the letter. The letters are deciphered as follows:
| ZZZ value | Decoding |
| X | Chromium |
| N | Nickel |
| WITH | Silicon |
| IN | Tungsten |
| M | Molybdenum |
| F | Vanadium |
| YU | Aluminum |
| G | Manganese |
| TO | Cobalt |
| T | Titanium |
All this sounds quite intimidating, but there is nothing complicated about it. Let's try to decipher several popular grades of high-alloy steel:
- А10Х13СУ - free-cut steel, which contains 0.10% carbon, 13% chromium, as well as silicon and aluminum in a concentration of less than 1%
- L12X17 - cast steel, which contains 0.12% carbon and 17% chromium.
- 12Х18Н12Т - steel that contains 0.12% carbon, 18% chromium, 12% nickel, as well as titanium in a concentration of less than 1%.
Designations by type
Structural steel of ordinary quality and not containing alloying elements according to the requirements of GOST 380-94 is designated by the letters “St” and, depending on the composition, by numbers from 0 to 6. Metal of higher quality receives a lower number. The letter “G” indicates a high proportion of Mn in the metal. The metal group is indicated before the brand itself.
The greater the presence of carbon in the metal and the strength of the steel, the greater the indicated figure. To indicate a subcategory of steel, a number is added to the grade sign at the end of a certain category; the first of them, as a rule, is not indicated. After indicating the type and grade number, the degree of deoxidation is indicated. For example, St1kp2 means:
- St – carbon of normal quality
- Marks are the first
- kp – boiling
- 2 – second category
Sometimes the markings are longer, for example, chemically resistant alloy steel 12Х18Н10Т. Explanation:
- The 12 at the beginning indicates the carbon content – 0.12%. In the absence of numbers, it is assumed that carbon is greater than 1%.
- X18 means that the chromium in the alloy is 18%
- H10 – 10% nickel
- T is titanium, the absence of a digital index means the mass fraction is less than 1%
Types and grades of high-alloy steel
| Steel category | Key Features | Brands of the corresponding category |
| Martensitic grades | They contain carbon in decent quantities (up to 0.7%), the chromium content is average (from 8 to 19%), and they contain silicon and/or manganese in small quantities. | 07Х16Н4Б, 13Х11Н2В2МФ, 30Х13 |
| Ferritic grades | Low carbon content (up to 0.15%), high to medium chromium content (12 to 30%), may contain silicon, titanium and/or manganese in very small quantities | 12Х17, 08Х13, 15Х25Т |
| Austenitic grades | Low carbon content (up to 0.2%), moderate to medium chromium content (from 10 to 18%), nickel in various concentrations (from 3 to 25%), manganese in various concentrations (from 1 to 14%), in small quantities may contain silicon, nitrogen | 20X25N20S2, 12X25N16G7AR |
| Composite martensitic-ferritic grades | Low carbon content (up to 0.2%), high or medium chromium content (from 10 to 16%), in small quantities - vanadium, manganese, silicon | 12Х13, 15Х12ВНМФ |
| Composite austenitic-ferritic grades | Low carbon content (no more than 0.18%), high chromium content (average 23%), manganese in various concentrations (there are alloys with both a low content of 0.5% and a high content of 9%), small inclusions are possible silicon, aluminum, titanium | 15Х18Н12С4ТУ, 12Х21Н5Т |
| Composite austenitic-martensitic grades | Carbon in various concentrations (from 0.1 to 1%), high chromium content (on average about 16%), in small concentrations - aluminum, silicon, titanium | 08Х17Н6Т, 09Х15Н8У1 |
Welding of high alloy steels
Welding high-alloy steels differs from welding conventional steels. The thing is that most high-alloy alloys have increased thermal conductivity and increased linear expansion of the metal, which forces a number of important changes to be made to the welding procedure:
- Increased thermal conductivity leads to excess heat collecting on the surface of the metal, which makes it much easier to melt the steel deeper. Therefore, when welding, it is necessary to reduce the welding current by 15-25% to avoid damage to the part.
- Due to the increased coefficient of expansion of the metal when heated, more severe deformation of the metal also occurs. When working with voluminous rigid volumetric structures, the risk of cracks also increases. Therefore, extreme care must be taken when welding.
Adviсe
In addition to this, there are many other features of welding high-alloy steels. When working with alloys that do not contain titanium or niobium, you need to remember the heating temperature of the welding arc. When the metal is heated to temperatures above 500 degrees, such alloys lose their anti-corrosion properties.
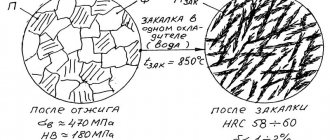
If, during welding, for some reason you brought the metal fragment to a temperature above 500 degrees, then in this case it is necessary to perform hardening or heat the fragment to a temperature of 850 degrees. In this case, alloying clusters dissolve and are evenly distributed throughout the alloy.
Due to the presence of alloying additives, the risk of steel cracking during welding increases significantly. To avoid this, you need to use electrodes coated with molybdenum, manganese or tungsten. When such tools are used, the seam area acquires a fine-grained structure, which prevents the formation of cracks.
Preheating the steel to a temperature of 100-300 degrees Celsius also reduces the risk of weld cracking. In this case, the heat will be evenly distributed throughout the entire thickness of the metal and prevent the formation of cracks.
Steel alloys with a carbon content of less than 0.12% must be heated before welding. If this is not done, then with a high degree of probability, after welding, cracks and corrosion build-ups will form at the weld site.