Stainless steels – chromium
Carbon and low-alloy steels are practically defenseless against corrosion in the atmosphere, in water and in most other environments. They are covered with a film of oxides, which does not have sufficient density and tightness to protect the steel from the chemical effects of the environment. At the same time, it is known that some alloying elements increase the resistance of steel against corrosion. These elements primarily include chromium and nickel.
When less than 12% chromium is added to steel, its corrosion resistance does not increase: it remains at the level of ordinary carbon steels. However, the introduction of more than 12% chromium into steel makes it resistant to corrosion in the atmosphere and in most other industrial environments. Steels with a chromium content of more than 12% are called corrosion-resistant or, as they are often called, stainless.
Three Types of Chromium Stainless Steels
Three types of chromium steels are used: with a nominal chromium content of 13, 17 and 25-28%. The composition of the main chemical elements of chromium steels according to GOST 5632-72 is presented in Table 1.
Table 1 - Composition of the main chemical elements of chromium corrosion-resistant steels according to GOST 5632-72
Chromium steels, depending on the carbon content, can belong to different structural classes: ferritic, martensitic and mixed - ferritic-martensitic. Belonging to one class or another is determined by the diagram of the iron-carbon-chromium ternary system.
Steels with a nominal chromium content of 17, 25 and 28% - 12Х17, 08Х17Т, 15Х25Т and 15Х28 - belong to the ferritic class steels. Their structure is ferrite and they do not undergo phase transformations.
For steels with a chromium content of 12-14%, everything is a little more complicated. They are unstable in properties and small deviations in the chemical composition transfer steel from one class to another. Thus, steel 08Х13 with a minimum carbon content and a maximum chromium content is ferritic, and with a minimum chromium content it has a gamma-alpha transformation.
Cooling steels 20Х13, 30Х13 and 40Х13 in air leads to the formation of martensite in them. The hardness of martensite increases with increasing carbon content, as well as the heating temperature for quenching, which determines the degree of dissolution of carbides in austenite.
Heat treatment of chromium stainless steels
Heat treatment of chromium steels can be different depending on the purpose pursued, the class of steel and its chemical composition. Commonly used heat treatment modes for chromium stainless steels are presented in Table 2
Table 2 - Typical heat treatment conditions for chromium stainless steels and their mechanical properties
Steel type X13
Steels with 13% chromium - 08Х13, 12Х13, 20Х13, 30Х13 and 40Х13 - are the most common and cheapest stainless steels. They are used for kitchen utensils and technology. Steels with low carbon content 08Х13 and 12Х13 have high ductility and various parts are stamped from them. Steels 20Х13, 30Х13 and 40Х13 have high hardness and increased strength - parts of increased strength and wear resistance with high corrosion resistance are made from them. They are used to make various instruments, including surgical ones, as well as bearings, springs and other parts for working in an active corrosive environment.
Steel type X17
Steels with 17% - 12Х17, 08Х17Т and 14Х17Н2 - chromium have higher corrosion resistance. Due to their higher chromium content, these steels are also used as heat-resistant (scale-resistant) at operating temperatures up to 900 °C.
Steel types X25-X28
Steels 15Х25Т and 15Х28 are used for furnace parts, for example, muffles and thermocouple covers, for operation at temperatures from 1050 to 1150 °C.
Problems with Ferritic Stainless Steels
A big disadvantage of ferritic stainless steels is their tendency to become coarse-grained when overheated, which cannot be eliminated by heat treatment - these steels do not undergo phase transformations. Coarse grain creates increased brittleness of steel with the transition of the cold brittleness threshold to the region of positive temperatures.
Source: Gulyaev A.P. Metallurgy,
steel-guide.ru
Structural alloy steels
marked using numbers and alphabetic abbreviations (for example, 15Х, 10Г2СД, 20Х2Н4А, etc.). The two-digit digital combination at the beginning of the brand displays the average C content in hundredths of a percent. The capital letter of the Russian alphabet indicates the name of the alloying element, in particular: B – (Nb), N – (Ni), Ф – (V), В – (W), М – (Mo), Х – (Cr), Г – (Mn), P – (P), C – (Zr), D – (Cu), P – (B), Ch – rare earth, E – (Se), C – (Si), Yu – (Al), K – (Co), T – (Ti), A – (N) only in the middle of the designation.
Numerical values after the letter abbreviation indicate the percentage of the alloying element. If there are no numbers, this means that the concentration of the alloying element is ≤ 1.5%.
The main volume of alloyed structural steels is smelted in the high-quality category (for example, 30ХГС).
If the letter “A” is located at the end of the brand name, this means that this steel is classified as a high-quality alloy steel (for example, 30KhGSA).
The presence of the letter “A” in the middle of the grade (for example, 16G2AF) indicates that this steel was also alloyed with nitrogen.
The letter “Ш” after the dash at the end of the brand name indicates that it belongs to the category of especially high-quality alloy steels (for example, 30ХГС-Ш, 30ХГСА-Ш).
If the structural alloy steel is cast, the letter “L” is added at the end of the grade designation (for example, 15GL, 40HNL, etc.).
Structural alloy chromium steels (0.6...1.6% Cr) are characterized by increased strength, hardness and ductility combined with high cold resistance. The presence of chromium also contributes to a decrease in elongation. Thus, the tensile strength of ordinary steel 40 is 580 MPa, the yield strength is 340 MPa, and the elongation rate is 19%. In chromium steel grade 40X, the values of similar indicators change, respectively, to 1000 MPa, 800 MPa and 13%. Such steels are indispensable in the production of shafts, gears, pushers, worm gears, hardware and other industrial products.
Structural steels alloyed with chromium
Chromium stainless steels
Search Lectures
The chromium content in them must be at least 12%. With a lower content, steel is not able to resist corrosion, because its electrode potential becomes negative. Steel achieves its greatest corrosion resistance after appropriate thermal (hardening + high tempering) and mechanical (grinding and polishing) treatments.
Steel has better resistance to corrosion only if the entire chromium content in the steel is in the solid solution. In this case, it forms a dense protective oxide film of Cr2O3 on the surface.
These steels must contain a small percentage of carbon, since an increase in the percentage of carbon leads to the formation of carbides, which reduce the amount of chromium in the solid solution and, accordingly, reduces corrosion resistance.
The most common grades of stainless chromium steels are:
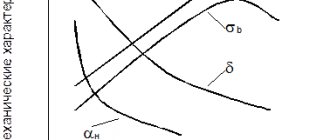
* 12X13 – martensitic steel, hardening from 1000-1100o in oil and tempering (700-750o) and polishing.
This steel is stable in mildly aggressive environments (water, steam), used for parts with increased plasticity (valves of hydraulic presses, household items) σв = 600-660 MPa δ = 16-20%
* 20Х13
* 30Х13-carburetor needles
* 40X13 – also martensitic class steel, used after hardening from 1000-1050o and tempering (180-200o) and grinding and polishing. These are surgical instruments, household cutting instruments, ball bearings operating in aggressive environments. 52-55НРСе.
* 12Х17 – more corrosion-resistant (acids and other environments), ferritic class.
* 15X28 is used for chemical and food equipment. However, it is not suitable for the manufacture of welded structures.
* When heated, the peripheral zone of the grains becomes depleted of chromium, which leads to intergranular corrosion. This is the most dangerous type of corrosion damage along the boundaries of austenite grains. To prevent it, titanium is introduced into steel. It binds carbon and eliminates the formation of chromium carbides.
* 08Х17Т – for welded structures.
Chrome-nickel stainless steels
They contain large amounts of chromium and nickel, little carbon and belong to the austenitic class. In addition to austenite, they contain chromium carbides.
* 12Х18Н9 - to obtain an austenitic structure, it is quenched in water from 1100-1150°C, and high corrosion resistance is achieved with relatively low strength. To increase its strength, steel is subjected to cold plastic deformation (hardening) and used in the form of a cold-rolled strip sheet. 17Х18Н9
Just like ferritic steel, due to the depletion of grains in chromium (chromium carbides are released), it is prone to intergranular corrosion. To prevent this, the steel is additionally alloyed with titanium or the percentage of chromium is reduced.
For example, 12Х18Н9Т or 04Х18Н9 - for the manufacture of chemical equipment
The great advantage of chromium-nickel steels of the austenitic class is their good manufacturability in relation to machine tool machining and welding.
* 12Х18Н10Т – used in cryogenic technology for transportation and storage of liquid gases, reservoirs, fuel tanks. But these steels are very expensive, so scarce nickel can be replaced with manganese: 10Х14Г14Н4Т
* 08Х22Н6Т
* 12Х21Н5Т – these are austenitic-ferritic steels, more durable than austenitic ones
* 09Х15Н8У – austenitic-martensitic class, high corrosion resistance + good mechanical properties + good weldability
High alloy acid-resistant steels
06ХН28МДУ – acid-resistant steel
For more severe conditions, nickel and copper alloys are used - Monel,
Nickel and chromium - Inconel
Nickel and molybdenum - Hastelloy
HEAT-RESISTANT STEEL
At high temperatures, alloys interact with the surrounding gaseous environment. What causes gas corrosion (oxidation) and destruction of the metal.
For the manufacture of structures operating at elevated temperatures (400-900°), special heat-resistant steels are used.
Heat resistance or scale resistance is generally understood as the ability of a material to resist corrosion damage under the influence of air and other gases at high temperatures.
Heat resistance is usually characterized by the temperature at which intense scale formation begins, when a thin film of oxides first forms on the surface of the steel, which increases over time and scale forms.
If the oxide film is porous, oxidation occurs intensively
If it is dense, oxidation slows down or even stops completely.
To obtain a dense (protective) oxide film, steel is alloyed with chromium, silicon or aluminum.
The degree of heat resistance depends on the amount of alloying elements.
* 15Х5chrome 5% - heat resistance up to 700° 12Х17 -chrome 17% - up to 900°
* 15Х28 - chrome 28% up to 1100-1150°
The higher the chromium content, the higher the heat resistance.
Important: That heat resistance, which depends so significantly on the composition, does not depend on the structure of the alloy.
Thus, the heat resistance of ferritic steels (pure chromium) and austenitic (chromium-nickel) steels is almost the same.
* For example, 12Х17 and 12Х18Н9 – their heat resistance is 900°
* Nickel-based alloys with chromium and aluminum have even greater heat resistance. ХН70У
search-ru.ru
What is the function of alloying elements in corrosion-resistant grades?
The main elements present in stainless steel:
- Chromium. Increases resistance to various types of corrosion, hardness, and strength. Slightly reduces ductility.
- Nickel. Increases corrosion resistance, strength characteristics, ductility, and hardenability.
- Manganese. When the content is more than 1%, it increases hardness, wear resistance, and resistance to sudden mechanical loads.
- Titanium. Increases strength, machinability, corrosion resistance, and refines grain.
- Niobium. Improves corrosion resistance of welds, increases the ability to contact acidic environments.
Chrome steels
Topics: Steel welding.
Chromium steels are not deficient in terms of alloying materials and are widely used for the manufacture of various types of equipment operating at high pressure and temperature under conditions of exposure to aggressive environments.
Alloying with chromium not only ensures the corrosion resistance of steels in oxidizing environments, but also determines their structure, mechanical properties, heat resistance, and technological properties. Forming a continuous series of solid solutions with iron at concentrations up to 12%, chromium then contributes to the closure of the γ-region, which is the main reason for the formation of different structures and the diversity of their properties in chromium steels.
Other related pages
Chrome steels
:
In accordance with the Fe - Cr diagram (Fig. 1), the γ-region is limited on the right by two lines closing the heterogeneous area
α(δ) + γ.
Chromium strongly influences the position of the critical points marking the α→γ transformation. Initially, an increase in chromium content leads to a decrease in point A3. At concentrations up to 8%, chromium is one of the elements that contribute to the stability of austenite and the expansion of its temperature range. Large concentrations of chromium increase point A3. For alloys with the α→γ transformation, alloying with chromium also significantly reduces the critical cooling rate. As a result, with low carbon content in chromium steels, the formation of a single-phase martensitic structure is possible. A clear example of this is the formation of martensite in the structure of X9M steel upon cooling from 800°C even at a very low (~1°C/s) rate.
| Figure 1. State diagram of Fe - Cr alloys. |
| Rice. 2. The influence of carbon on the closure of the γ-region in Fe-Cr alloys. |
In carbon-free alloys, the γ-region closes at 11 ... 12% Cr. At higher chromium contents, austenite occurs only in alloys with a high carbon content (Fig. 2). At a content of -12% Cr in low-carbon steels after cooling, along with martensite, a certain amount of ferritic component is found in the structure.
At >12% Cr, all carbon-free alloys are ferritic.
Chromium effectively increases the corrosion resistance of steels at concentrations >12%. Therefore, less alloyed chromium steels are used mainly as a structural material for highly loaded energy and petrochemical equipment. Equipment for working in aggressive liquid and gaseous environments (sea water, acids, fuel combustion products, etc.) is made from steels with ≥13% Cr. Possessing high corrosion resistance, high-alloy chromium steels of the ferritic class are not suitable for equipment operating under high-temperature creep conditions. This is due to the low heat resistance caused by the ferritic structure of high-chromium steels.
Joint alloying with chromium and nickel contributes to the formation of homogeneous and heterophase structures in steels, the formation, along with martensite and ferrite, of an austenitic component, the amount of which depends on the concentration of the above elements. The structure of chromium steels additionally alloyed with nickel can be assessed using a Scheffler diagram (see Figure 1 on the Austenitic steels page). This diagram also allows you to calculate the effect on the structure of other alloying elements.
In accordance with GOST 5632-72, high-alloy steels are divided into groups: corrosion-resistant, heat-resistant and heat-resistant.
Depending on the structure, chromium steels can be classified into different classes:
martensitic, martensitic-ferritic, ferritic, austenitic-martensitic and austenitic-ferritic
- < Martensitic steels
- Properties of carbon steel >
weldzone.info
Bibliography
- Medovar L.B. Modern rolls of rolling mills. Requirements, materials and production methods / L.B. Medovar, A.K. Tsykulenko, V.E. Shevchenko // Problems of SEM.-2001.-No. 1.- P. 38-48.
- Tolstov I.A. Improving the performance of hot deformation tools / I.A. Tolstov, A.V. Pryakhin, V.A. Nikolaev. -M.: Metallurgy, 1990.- 143 p.
- Formation of wear-resistant structures of chromium steels and chavuns / V.I. Tikhonovich, V.P. Gavrilyuk, V.V. Tikhonovich, A.N. Grinachevsky // Metal science and processing of metals. - 2003. - No. 3. - P. 16-23.
- Features of the structure of promising materials for hot rolling rolls / V.V. Pashinsky, A.D. Ryabtsev, V.V. Gorbatenko, E.G. Pashinskaya // Steel. - 2003. - No. 5. - P. 73-75.
- Grabovsky V.Ya. Austenitic die steels and alloys for hot deformation of metals / V.Ya. Grabovsky, V.I. Buzzard // MiTOM.- 2001.- No. 10.- P. 31-34.
- Pashinsky V.V. Structure and properties of high-carbon tool steel with increased resistance to supercooled austenite / V.V. Pashinsky, V.V. Gorbatenko // Construction, materials science, mechanical engineering: Collection of scientific papers. Issue 26, part 2. - Dnepropetrovsk: RIA "Dnepr - VAL". - 2004. - P. 90-95.
- Artinger I. Tool steels and their heat treatment / I. Artinger; lane from Hungarian. - M.: Metallurgy, 1982. - 312 p.
Reviewer: Ph.D., prof. N.T. Egorov
© V.P. Gorbatenko, V.V. Gorbatenko, V.V. Pashinsky, V.G. Konarev
- ← Features of silicon base technology for producing polycrystalline silicon
- Technology of applying wear-resistant chromium nitride coatings to stamping tools using magnetron sputtering →
Chromium steel - grade - Great Encyclopedia of Oil and Gas, article, page 1
Chromium steel - grade
Page 1
Chromium steel grades 0X13; ОХ17Т and Х25Т are cheaper, but they are poorly welded and their use for devices subject to control by the State Technical Supervision Authority is not allowed. [2]
Chromium steel grades X5, 1X13, 2X13, 3X13 and EI496 are widely used in apparatus for processing high-sulfur oils: X5 for the manufacture of pipes for heat exchange and condensation refrigeration equipment; X5M - for the manufacture of furnace pipes, forgings, furnace twins, flanges and other equipment operating at temperatures of about 600 - 630 C. Chrome-nickel steels of the grades OX18N9, 1X18N9, 1X18N10T and EI496 are characterized by heat resistance, heat resistance and high corrosion resistance in many aggressive environments. [3]
Chromium steel grades SKh6M, SKH6, ESH8, Kh5M, Kh5MF, 1X13 and others with a chromium content of 4 to 14% belong to the martensitic class. Steels Kh28, 1Kh17Yu5 and others with a chromium content from 18 to 30% belong to the ferritic class. These steels resist oxidation well at high temperatures. [4]
Chromium steel grades SKh6M, SKH6, ESH8, Kh5M, Kh5MF, 1X13 and others with a chromium content of 4 to 14% belong to the martensitic class. Steels Kh28, 1Kh17Yu5 and others with a chromium content from 18 to 30% belong to the ferritic class. These steels resist oxidation well at high temperatures. [5]
Chromium steel grades X25 and X28 do not corrode in nitric acid even at boiling point. Only very strong (smoking) nitrogen vapor destroys steel of these grades. [6]
Chromium steel grade X5M is used as a corrosion-resistant and heat-resistant (up to 650) steel in the oil processing industry (pipes, cracking plants) and in boiler-turbine construction for parts operating at high pressure and elevated temperature. Silchrome grade X12YUS can be used as a material for cementation boxes. [7]
Chromium steels of grades Kh6SM, 4Kh9S2, Kh5M, Kh5MF, 1X13 (EZh-1) and others with a chromium content of 4 - 14% belong to the martensitic class. [8]
Chromium steel grade 9X differs from tool carbon steel by the presence of a chromium additive. 9X steel provides minimal deformation during hardening and is used primarily for the manufacture of nuts and machine taps. [9]
Chromium steel grades 1X13 and 2X13 are welded with Sv-10Kh13 or Sv-06Kh14 wires using fluxes AN-26, FTsL-2, ANF-5, etc. You can also use ceramic flux KhNK-66. Metal up to 10 mm thick is welded without preheating. For large thicknesses, preliminary and accompanying heating to 250 - 300 C is used. After welding, tempering is carried out at 680 - 700 C. [10]
Chromium steel grades 0X13, 1X13, 2X13 and 3X13 are resistant to atmospheric conditions and in low-aggressive solutions; with increasing carbon content, they are hardened, are characterized by increased strength, and are heat-resistant up to a temperature of 750 C. Chromium steels of grades X17 and OX17T are resistant to oxidizing agents (for example [11]
Chromium steel grades ZOX-50X is widely used in the oil, petrochemical and gas industries. Thus, steel grades ZOX and 35X are used to make studs and bolts for flange connections in oil refinery installations, where the ambient temperature is not higher than 450 C for open flanges and 400 C for insulated flanges. Steel grade 38ХА is used for the manufacture of turbodrill parts, housings, nipples, subs, and shafts. [12]
Chromium steel grades ZOX-50X is widely used in the oil, petrochemical and gas industries. Thus, steel grades ZOX and 35X are used to make studs and bolts for flange connections in oil refinery installations, where the ambient temperature is not higher than 450 C for open flanges and 400 C for insulated flanges. [14]
Chromium steel grade X (ШХ15) (Table 30) after quenching in oil receives a hardness of Rc 62 - b64, which is maintained when tempered to 170, so this steel is used for measuring instruments, drills and reamers. Along with high hardness, this steel has low deformation during hardening. [15]
Pages: 1 2 3 4
www.ngpedia.ru
Chrome steel is... What is Chrome steel?
Chromium, like carbon, has the property of significantly increasing the hardness of steel and increasing its elastic limit. This effect is already detected at a chromium content of 1%; with the greatest intensity it manifests itself with a chromium content of 2 - 2 ½%. When testing specimens made of hardened steel for tensile strength, they sometimes exhibit resistance of up to 140 kg per square meter. mm, moreover, at a greater elongation than ordinary steel samples with the same carbon content. Having a fracture in an unhardened state that is no different from that of ordinary carbon steel, X. steel, after hardening, takes on an extremely fine-grained fracture with a silky shine. The hardness itself penetrates into its mass more deeply than in ordinary steel. In X. processing, steel is very hard, so it cannot be used for the manufacture of products that require processing with cutting tools. But cutters made from it are distinguished by extraordinary durability and can cut the hardest metals. However, with a high chromium content, the edges of cutters made of X. steel are easily chipped; Therefore, the chromium content in tool steel is rarely increased above 1.5%. A noticeable disadvantage of X. steel is its complete inability to weld, explained by the fact that chromium oxide is very refractory. In small doses, an admixture of chromium increases the strength of iron without causing its brittleness, which is why chromium is often mixed even with ordinary cast iron intended for construction work. Products made of X. steel require careful annealing, which brings the elastic limit, increased by hardening, to its normal value. The chromium admixture has no effect on the formation of shrinkage cavities. — Heating X. steel during its processing should be done with special care, since it is much stronger than ordinary carbon steel, it tends to burn out, and in the burnt state it takes on a coarse-grained fracture and becomes very brittle. Adding X. iron to a molten bath in an open-hearth furnace, in which the metal is exposed to atmospheric oxygen, is extremely difficult, since chromium greedily combines with oxygen. Therefore, the best grades of X. steel are produced by crucible melting, in hermetically sealed crucibles. The first experiments on X. steel were made in 1875 by a French plant in Unieux (Loire), and since 1877 this plant began to produce X. steel in factory sizes. In general, and to this day, the main producer of X. steel for all of Europe is France. The uses of X. steel are quite varied. It is used to make: artillery shells, dugout sheets, springs, etc. Its main use is to make all kinds of cutting tools from it. Metallurgical plants usually produce three types of X. tool steel: 1) very hard, 2) hard and 3) tough. From the first variety, cutters are made for processing hardened cast iron, hardened projectiles, wheel tires hardened by friction on rails, hard rocks, etc. Such cutters are heated without tempering or with tempering to a straw-yellow color. They should not be subjected to impacts, otherwise they will easily chip. The second type of steel is used for the production of ordinary cutting tools for turning, planing, drilling, etc., used in normal use in mechanical factories. Finally, its third variety is used for the manufacture of such tools that must experience strong blows and shocks, such as: chisels, hole-punching punches, stamps, boiler chocks, etc. It is excellently hardened and when hardened, with great hardness, it differs and high viscosity, which is why cutters made from it stand for a very long time without refueling. “Recently, however, opinions have begun to be expressed regarding the exaggeration of the effect on steel attributed to chromium. This opinion was expressed, by the way, by Prof. Ledebour, based on the experiments of Gove, who, having tested 12 samples of X. steel, found that 6 of them had an absolute strength, not greater than that of ordinary steel; 3 samples showed even lower strength, and only 3 others turned out to be slightly stronger than ordinary steel with the same carbon content. The selling price of X. steel is significantly higher than ordinary steel.
V. S. Knabbe. Δ.
dic.academic.ru
How to decipher the markings
Marking of stainless steels, the rules for the formation of which are stipulated by the provisions of regulatory documents, carries the following information:
- the number in first place indicates the quantitative content of such a chemical element as carbon in the alloy composition (for example, in steel grade 08Х17 carbon is contained in an amount of 0.08%, and in 40Х13 - 0.4%);
- after the letters in the marking, each of which indicates the corresponding chemical element (X - chromium, H - nickel, M - manganese), numbers are placed indicating its content in whole percentages.
An example of decoding the designation of stainless steel
In general, if we talk about the rules for marking steel alloys classified as stainless, they are practically no different from those adopted for marking steels of any other type.
chromium steel is... What is chromium steel?
chromium steel
ua\\ chromium steel
en\ \ chromium steel
de\\Chromstahl, chromlegierter Stahl
fr\\\\acier au chrome
Terminological dictionary "Metals". — Moscow-Zaporozhye: Motor Sich. 2005.
See what “chromium steel” is in other dictionaries:
- CHROME STEEL - (Chrome steel) steel, which contains chromium (2 5%). X.S. has greater hardness, and with a high chromium content (12-15%) it has high resistance to corrosion by acids. Samoilov K.I. Marine dictionary. M.L.: State... ... Marine Dictionary
- chromium steel - chrominis plienas statusas T sritis chemija apibrėžtis Fe lydinys, turintis 0.70–1.65% Cr ir 0.15–1.10% C. atitikmenys: angl. chromium steel rus. chromium steel … Chemijos terminų aiškinamasis žodynas
- Chromium steel - Chromium, like carbon, has the property of significantly increasing the hardness of steel and increasing its elastic limit. This effect is already detected at a chromium content of 1%; with the greatest intensity it manifests itself when the chromium content is 2... ... Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron
- Steel - This term has other meanings, see Steel (meanings). Steel Phases of iron-carbon alloys Ferrite (solid solution of interstitial C in α iron with a body-centered cubic lattice) Austenite (solid solution of interstitial C in γ ... ... Wikipedia
- Steel types - see Raw (pudding, welding) steel, Cast steel, Classification of iron products, Hardening, Cementation, Steel microstructure, Armor plates, Damask steel, Rolling rollers. For some special types of steel, in addition, see Nickel,... ... Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron
- Steel, its types - see Raw (pudding, welding) steel, Cast steel, Classification of iron products, Hardening, Cementation, Microstructure of steel, Armor plates, Damask steel, Rolling rollers. For some special types of steel, in addition, see Nickel,... ... Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron
- Steel chemical analysis* - The methods of chemical analysis for steel, cast iron and iron are almost exactly the same; therefore, here we will indicate methods for analyzing various types of iron in general, and not specifically one S. Iron analyzes are among the most difficult, due to the greater... ... Encyclopedic Dictionary of F.A. Brockhaus and I.A. Efron
- Steel, chemical analysis—Methods of chemical analysis for steel, cast iron, and iron are almost exactly the same; therefore, here we will indicate methods for analyzing various types of iron in general, and not specifically one S. Iron analyzes are among the most difficult, due to the greater... ... Encyclopedic Dictionary of F.A. Brockhaus and I.A. Efron
- STAINLESS STEEL - steel that is resistant to corrosion in air. atmosphere, sea and speech water, as well as in certain aggressive environments. The most common are chromium-nickel (18% chromium and 9% nickel) and chromium (13-27% chromium) steels, often with the addition of other elements, for example... ... Large Encyclopedic Polytechnic Dictionary
- Corrosion-resistant steel is steel that is resistant to corrosion in air, sea and river water, as well as in some aggressive environments at different temperatures. The most common are chromium-nickel (18% Cr, 9% Ni) and chromium (13-27% Cr) steels, often with the addition of... ... Encyclopedic Dictionary of Metallurgy
- CORROSION-RESISTANT STEEL - steel that is resistant to corrosion in air, sea and river water, as well as in some aggressive environments at different temperatures. The most common are chromium-nickel (18% Cr, 9% Ni) and chromium (13-27% Cr) steels, often with the addition of other ... Metallurgical Dictionary
metals_ru_uk.academic.ru
Physical properties and mechanical characteristics of chromium metal and its compounds
Chrome is not a structural material, but is used quite widely due to the fact that it has excellent anti-corrosion properties. Chrome plating protects any other alloy from rust. In addition, alloying steels with chromium gives them the same resistance to corrosion that is characteristic of the metal itself.
So, let's discuss today what are the technical and oxidation characteristics of the chromium material, the main amphoteric, reducing properties and metal production will also be affected. We will also find out what the effect of chromium is on the properties of steel.
Chromium is a metal of period 4 of group 6 of the secondary subgroup. Atomic number 24, atomic mass - 51.996. It is a hard metal with a silvery-bluish color. In its pure form it is malleable and tough, but the slightest admixtures of nitrogen or carbon give it brittleness and hardness.
Chromium is often classified as a ferrous metal due to the color of its main mineral, chromium iron ore. But it got its name from the Greek “color”, “paint”, thanks to its compounds: metal salts and oxides with varying degrees of oxidation are painted in all the colors of the rainbow.
- Under normal conditions, chromium is inert and does not react with oxygen, nitrogen or water.
- In air, it is immediately passivated - covered with a thin oxide film, which completely blocks oxygen from accessing the metal. For the same reason, the substance does not interact with sulfuric and nitric acid.
- When heated, the metal becomes active and reacts with water, oxygen, acids and alkalis.
It is characterized by a body-centered cubic lattice. There are no phase transitions. At a temperature of 1830 C, a transition to a face-centered lattice is possible.
However, chromium has one interesting anomaly.
At a temperature of 37 C, some physical properties of the metal change sharply: electrical resistance and linear expansion coefficient change, the elastic modulus drops to a minimum and internal friction increases.
This is due to the passage of the Néel point: at this temperature, the substance changes its antiferromagnetic properties to paramagnetic ones, which represents a first-level transition and means a sharp increase in volume.
The chemical properties of chromium and its compounds are described in this video:
The physical characteristics of a metal are affected by impurities to such an extent that even the melting point has proven difficult to determine.
- According to modern measurements, the melting point is considered to be 1907 C. The metal is a refractory substance.
- The boiling point is 2671 C.
Below we will give a general description of the physical and magnetic properties of chromium metal.
General properties and characteristics of chromium
Physical Features
Chromium is one of the most stable of all refractory metals.
- Density under normal conditions is 7200 kg/cubic meter. m, this is less than that of iron.
- Hardness on the Mohs scale is 5, on the Brinell scale 7–9 Mn/m2. Chromium is the hardest metal known, second only to uranium, iridium, tungsten and beryllium.
- The elastic modulus at 20 C is 294 GPa. This is a fairly moderate figure.
Due to its structure - a body-centered lattice, chromium has such a characteristic as the temperature of the brittle-ductile period.
But when it comes to this metal, this value turns out to be highly dependent on the degree of purity and ranges from -50 to +350 C.
In practice, crystallized chromium does not possess any ductility, but after gentle annealing and forming it becomes malleable.
The strength of the metal also increases with cold working. Alloying additives also significantly enhance this quality.
The following is a brief description of the thermophysical properties of chromium.
Thermophysical characteristics
As a rule, refractory metals have a high level of thermal conductivity and, accordingly, a low coefficient of thermal expansion. However, chromium differs noticeably in its qualities.
At the Néel point, the coefficient of thermal expansion makes a sharp jump, and then continues to increase noticeably with increasing temperature. At 29 C (before the jump), the value of the coefficient is 6.2 · 10-6 m/(m•K).
Thermal conductivity obeys the same pattern: at the Néel point it falls, although not so sharply and decreases with increasing temperature.
- Under normal conditions, the thermal conductivity of the substance is 93.7 W/(m•K).
- The specific heat capacity under the same conditions is 0.45 J/(g•K).
Electrical properties
Despite the atypical “behavior” of thermal conductivity, chromium is one of the best conductors of current, second only to silver, copper and gold in this parameter.
- At normal temperature, the electrical conductivity of the metal will be 7.9 · 106 1/(Ohm•m).
- Specific electrical resistance – 0.127 (Ohm•mm2)/m.
Up to the Néel point - 38 C, the substance is antiferromagnet, that is, under the influence of a magnetic field and in its absence, no magnetic properties appear. Above 38 C, chromium becomes paramagnetic: it exhibits magnetic properties under the influence of an external magnetic field.
In nature, chromium is found only in bound form, so the entry of pure chromium into the human body is excluded. However, it is known that metal dust irritates lung tissue and is not absorbed through the skin. The metal itself is not toxic, but the same cannot be said about its compounds.
- Trivalent chromium is released into the environment when chromium ore is mined and processed. However, it can also enter the human body as part of a dietary supplement - chromium picolinate, used in weight loss programs. As a microelement, the trivalent metal is involved in the synthesis of glucose and is essential. Excess of it, judging by research, does not pose a certain danger, since it is not absorbed by the intestinal walls. However, it can accumulate in the body.
- Hexavalent chromium compounds are more than 100–1000 times toxic. It can enter the body during the production of chromates, during chrome plating of objects, and during some welding operations. Compounds of the hexavalent element are strong oxidizing agents. Once in the gastrointestinal tract, they cause bleeding of the stomach and intestines, possibly with perforation of the intestine. The substances are almost not absorbed through the skin, but have a strong corrosive effect - burns, inflammation, and ulcers are possible.
The compound has the same effect on the respiratory system, but given the greater sensitivity of the mucous membrane, the picture here is more destructive.
Chromium is a mandatory alloying element in the production of stainless and heat-resistant steels. Its ability to resist corrosion and transfer this quality to alloys remains the most sought-after quality of the metal.
The chemical properties of chromium compounds and its redox properties are discussed in this video: