ore mining process
Hydrometallurgy
This is a technique in the field of mining metallurgy, the extraction of metals from their ores. Hydrometallurgy involves the use of aqueous solutions to extract metals from ores, concentrates and secondary or residual materials.[1][2] Technologies complementary to hydrometallurgy: pyrometallurgy, steam metallurgy and molten salt electrometallurgy. Hydrometallurgy is usually divided into three main areas:
- Leaching
- Concentration and purification of the solution
- Recovery of metals or metal compounds
Leaching
Leaching involves the use of aqueous solutions to extract metal from metal-containing materials that are in contact with material containing the valuable metal.[3] The first examples come from Germany and Spain in the 17th century, where it was used for copper mining.[4]
In leach solution conditions are varied in terms of pH, redox potential, presence of chelating agents and temperature to optimize the rate, extent and selectivity of dissolution of the desired metal component in the aqueous phase. Through the use of chelating agents, it is possible to selectively extract certain metals. Such chelating agents are typically Schiff base amines.[5]
The five basic leach reactor configurations are: in-situ, heap, vat, tank, and autoclave.
Leaching in place
In situ leaching is also called “solution mining.” Initially, the process involves drilling holes into the ore deposit. Explosives or hydraulic fracturing are used to create open pathways within the sediment for solution to penetrate. The leach solution is pumped into the deposit where it comes into contact with the ore. The solution is then collected and processed. The Beverly Uranium Mine is an example of in situ leaching, as well as the Trojan Mine in Zimbabwe.[ citation needed
]
Heap leaching
In heap leaching processes, crushed (and sometimes agglomerated) ore is piled into a pile covered with an impervious layer. The leach solution is sprayed on top of the pile and allowed to trickle down through the pile. The dump design typically includes settling tanks that allow the "saturated" leach solution (i.e., the solution with dissolved valuable metals) to be pumped for further processing. An example is gold cyanidation, where pulverized ores are extracted with a solution of sodium cyanide, which in the presence of air dissolves the gold, leaving a non-precious residue.
Balloon model of the complex anion aurocyanide or dicyanoaurate (I), [Au(CN)2]−.[6]
Leaching VAT
VAT leaching involves contacting the material, which is usually crushed and classified, with a leaching solution in large vats.
Tank Leaching
Agitated tank, also called stirred tank leaching, involves contacting material that is typically crushed and classified with a leach solution in agitated tanks. Stirring can improve reaction kinetics by increasing mass transfer. Reservoirs often have a series reactor configuration.
Leaching in an autoclave
Autoclave reactors are used for reactions at higher temperatures, which can increase the reaction rate. Likewise, autoclaving allows the use of gaseous reagents in the system.
Corrosion
Corrosion is a process of spontaneous destruction of alloys and metals that occurs under the influence of the environment. Rust begins to appear when exposed to oxygen, water, sulfur oxides, and carbon.
Types of corrosion:
- atmospheric.
- electrolytic;
- gas;
- lifting;
- biological.
It is impossible to imagine human life without metals. They are used in various fields of activity. The process of mining metal ore to make homogeneous materials or alloys has remained virtually unchanged for hundreds of years. New equipment and technology appeared, but the essence of the process remained the same.
Concentration and purification of the solution
After leaching, the leach liquor usually must be concentrated with metal ions to be recovered. In addition, removal of unwanted metal ions is sometimes required.[1]
- Precipitation is the selective removal of a target metal compound or the removal of a major impurity by precipitation of one of its compounds. Copper is precipitated as sulfide to purify nickel leaching products.
- Cementation is the conversion of a metal ion into a metal through an oxidation-reduction reaction. Typical applications include adding scrap metal to a copper ion solution. The iron dissolves and metallic copper is deposited.
- Solvent extraction
- Ion exchange
- Gas reduction. Treatment of a solution of nickel and ammonia with hydrogen produces metallic nickel in powder form.
- Electrowinning is especially selective if the electrolysis process used to separate the precious metals is expensive. Gold can be galvanized from its solutions.
Solvent extraction
Solvent extraction is a mixture of an extractant and a diluent used to extract a metal from one phase to another. In solvent extraction, the mixture is often called "organic" because the main component (diluent) is some type of oil.
PLS (saturated leach solution) is mixed until emulsified with the removed organic matter and allowed to separate.[ citation needed
] The metal will be replaced by PLS with the organic they modified.[
clarification needed
] The resulting streams will be loaded organic and raffinate.
In electrolytic separation, the loaded organics are then emulsified with the depleted electrolyte and allowed to separate. The metal will be replaced by organics and electrolyte. The resulting streams will be organic-free and electrolyte-rich. The organic stream is recycled through the solvent extraction process while the aqueous streams are cycled through the leaching and electrochemical recovery[ clarification needed
] processes respectively.[
citation needed
]
Ion exchange
Chelating agents, natural zeolite, activated carbon, resins and liquid organics impregnated with chelating agents, are used to exchange cations or anions with the solution.[ citation needed
] Selectivity and recovery depend on the reagents used and the contaminants present.
Properties of ores
Answering the question: what properties does iron ore have is not entirely simple. If only because the list of properties depends on the percentage of a given metal in the ore and the amount of foreign impurities. For example, red iron ore containing hematite (Fe2O3) contains as much as 70% of the total iron.
In general, by the way, only those where the ores contain 40% iron or more are considered viable iron mining. This figure really makes it clear that iron is widespread in the surrounding world many times more than other elements. For example, for the same uranium, its content in the ore in the amount of 2% would be considered an unprecedented success...
But let's return to our red iron ore. When characterizing iron ore, we can say that red iron ore ranges from a powdery substance to a dense one.
Limonite (also known as brown iron ore) is also an iron ore, but it is a porous and friable rock containing significant proportions of phosphorus and manganese. Clay is often used as waste rock. Due to this, by the way, it is quite easy to extract iron. That’s why cast iron is often made from it.
Metal recovery
Metal recovery is the final stage of the hydrometallurgical process. Metals suitable for sale as raw materials are often produced directly from the metal extraction stage. However, sometimes further refining is required if ultra-high purity metals are to be obtained. The main types of metal reduction processes are electrolysis, gas reduction and precipitation. For example, the main target of hydrometallurgy is copper, which is usually obtained by electrolysis. Cu2+ ions are reduced at moderate potentials, leaving behind other contaminant metals such as Fe2+ and Zn2+.
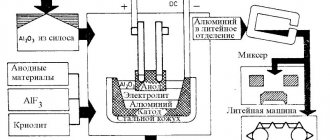
Electrolysis
Electrowinning and electrorefining, respectively, involve the extraction and purification of metals using metal electrodeposition at the cathode, or metal dissolution or competitive oxidation reaction at the anode.
Precipitation
Precipitation in hydrometallurgy involves the chemical precipitation of either metals and their compounds or contaminants from aqueous solutions. Precipitation will continue when, through reagent addition, evaporation, pH change or temperature manipulation, any particular species exceeds its solubility limit.
Description
These are chemical processes occurring in metallurgical units at high (800-2000°C) temperatures. Therefore, pyrometallurgy is sometimes called “high temperature chemistry.” Often chemical reactions are accompanied by a change in the aggregate state of the reacting substances: melting, sublimation, evaporation of the resulting metals or their compounds. In such processes, interactions can occur between solid, liquid (melts) and gaseous phases in any combination.
Pyrometallurgical processes are the processes of agglomeration of metallurgical raw materials, smelting of charge materials, production of alloys, and refining of metals. In particular, this is roasting, blast furnace smelting, open-hearth smelting, smelting in converters, arc and induction furnaces. Pyrometallurgy is the basis for the production of cast iron, steel, lead, copper, zinc, etc.
In pyrometallurgy, carbon reduction is often used - in cases where the metals being reduced do not form stable carbides, in addition to those mentioned above, such metals include germanium, cadmium, tin and others. In cases where stable carbides are formed by reduced metals, metallothermy is often used instead of reduction with carbon.
Pyrometallurgy is the main and most ancient field of metallurgy. From ancient times until the end of the 19th century, metal production was based almost exclusively on pyrometallurgical processes. At the turn of the 19th and 20th centuries, another major branch of metallurgy, hydrometallurgy, acquired industrial importance. However, pyrometallurgy continues to maintain a dominant position both in terms of production scale and variety of processes.
At the beginning of the 20th century, along with flame heating methods, different types of electric heating (arc, induction, etc.) began to be used in metallurgy; Around the same time, electrolysis of molten chemical compounds was introduced into industry (production of aluminum and other non-ferrous metals).
In the 2nd half of the 20th century, plasma melting of metals, zone melting and electric fire melting became widespread. Metallurgical processes based on the use of electric current are classified as an independent field of pyrometallurgy - electrometallurgy.
Recommendations
- ^ a b
Brent Hiskey "Metallurgy, a Review" in Kirk-Othmer Encyclopedia of Chemical Technology, 2000, Wiley-VCH, Weinheim. Doi:10.1002 / 0471238961.1921182208091911.a01 - F. Habashi "Recent Trends in Mining Metallurgy" Journal of Mining and Metallurgy, Section B: Metallurgy, 2009, Vol. 45, pp. 1-13. doi:10.2298/JMMB0901001H
- Um, Namil (July 2022). Hydrometallurgical Process for Extracting Rare Earth Elements from Waste: Basic Application of Acid Leaching with Designed Scheme
. INTEC. pp. 41–60. ISBN 978-953-51-3402-2. - Habashi, Fathi (2005). "A Brief History of Hydrometallurgy". Hydrometallurgy
.
79
(1–2): 15–22. doi:10.1016/j.hydromet.2004.01.008. - Tasker, Peter A.; Tong, Christine S.; Westra, Arjan N. (2007). "Co-extraction of cations and anions in the extraction of non-ferrous metals." Coordination Chemistry Reviews
.
251
(13–14): 1868–1877. doi:10.1016/j.ccr.2007.03.014. - Greenwood, N.N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.), Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.