Pyrometallurgy
Pyrometallurgy
is a set of metallurgical processes occurring at high temperatures. This is a branch of metallurgy associated with the production and purification of metals and metal alloys at high temperatures, in contrast to hydrometallurgy, which involves low-temperature processes.
Description[ | ]
These are chemical processes occurring in metallurgical units at high (800-2000°C) temperatures. Therefore, pyrometallurgy is sometimes called “high temperature chemistry.”
Often chemical reactions are accompanied by a change in the aggregate state of the reacting substances: melting, sublimation, evaporation of the resulting metals or their compounds.
In such processes, interactions can occur between solid, liquid (melts) and gaseous phases in any combination.
Pyrometallurgical processes are the processes of agglomeration of metallurgical raw materials, smelting of charge materials, production of alloys, and refining of metals. In particular, this is roasting, blast furnace smelting, smelting in converters, arc and induction furnaces. Pyrometallurgy is the basis for the production of cast iron, steel, lead, copper, zinc, etc.
In pyrometallurgy, carbon reduction is often used - in cases where the metals being reduced do not form stable carbides, in addition to those mentioned above, such metals include germanium, cadmium, tin and others. In cases where stable carbides are formed by reduced metals, metallothermy is often used instead of reduction with carbon[1].
Pyrometallurgy is the main and most ancient field of metallurgy. From ancient times until the end of the 19th century, metal production was based almost exclusively on pyrometallurgical processes.
At the turn of the 19th and 20th centuries, another major branch of metallurgy, hydrometallurgy, acquired industrial importance.
However, pyrometallurgy continues to maintain a dominant position both in terms of production scale and variety of processes.
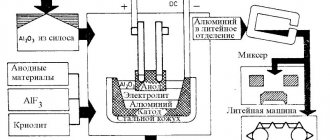
At the beginning of the 20th century, along with flame heating methods, different types of electric heating (arc, induction, etc.) began to be used in metallurgy; Around the same time, electrolysis of molten chemical compounds was introduced into industry (production of aluminum and other non-ferrous metals).
In the 2nd half of the 20th century, plasma melting of metals, zone melting, etc. became widespread. Metallurgical processes based on the use of electric current are classified as an independent field of pyrometallurgy - electrometallurgy.
Basic processes[ | ]
The main process of pyrometallurgy is ore smelting, which is carried out at such high temperatures that the products of chemical interaction melt, forming two liquid phases - metal or sulfide and slag. There are reduction and oxidation melts.
The defining process of reduction smelting is the reduction of metal oxides to ultimately produce a molten metal or its alloy with other elements. A typical reduction smelting process is the production of pig iron in blast furnaces. Reduction processes are also the main ones in the smelting of manganese, oxidized nickel, lead, and titanium ores.
The main reducing agents are carbon, carbon monoxide and hydrogen. Carbon monoxide is formed in the furnace itself when carbon is not completely burned; The main amount of hydrogen is obtained as a result of the decomposition of natural gas blown into the furnace.
A type of reduction smelting is the metallothermic production of metals, in which another metal with a greater affinity for oxygen is used as a reducing agent for a certain metal (Mn, Cr, V, etc.): Ca; Mg; Al and also Si. One of the advantages of metallothermic reduction is the production of metals that are not contaminated by carbon or hydrogen.
A typical oxidative ore smelting process is the processing of rich copper sulfide ores in shaft furnaces. During smelting, the bulk of the sulfur in sulfide minerals is oxidized, resulting in the release of a significant amount of heat. The main target product of smelting is the melt of FeS and Cu2S sulfides - matte.
Pig iron and ore smelting matte are essentially intermediate products that require additional processing. This treatment involves blowing the melts with air or pure oxygen, as a result of which the impurities contained in the alloys are oxidized and pass either into slag (SiO2; MnO; FeO, etc.) or into gas (CO; SO2). The process is called conversion.
A similar process to conversion is the fumming process—blowing slag melts with gas. Its difference from converting is that the metal melt is blown with an oxidizing gas, and when fumigating the slag, with a reducing gas.
And secondly, the products of oxidation of the metal melt - metal oxides - form a second liquid phase - slag, and the products of slag fuming - reduced volatile metals (or sulfides) in a vapor state are removed from the reaction space by a gas flow [2].
Literature[ | ]
Alloys
Metals easily form alloys - materials that have metallic properties and consist of two or more chemical elements (simple substances), at least one of which is a metal. Many metal alloys have a single metal as the base with small additions of other components. In principle, it is difficult to draw a clear boundary between metals and alloys, since even the purest metals contain “trace” impurities of other chemical elements.
All the items listed above - machines, airplanes, cars, frying pans, forks, spoons, jewelry - are made from alloys. Impurity metals (alloying components) very often change the properties of the base metal for the better, from a human point of view. For example, both iron and aluminum are fairly soft metals. But when combined with each other or with other components, they turn into steel, duralumin and other durable structural materials. Let's look at the properties of the most common alloys.
Steel is an alloy of iron and carbon, containing up to 2% of the latter. Alloy steels also contain other chemical elements - chromium, vanadium, nickel. There are far more steels produced than any other metals and alloys, and it is difficult to list all of their possible uses. Low-carbon steel (less than 0.25% carbon) is consumed in large quantities as a structural material, and steel with a higher carbon content (more than 0.55%) is used to make cutting tools: razor blades, drills, etc.
Iron forms the basis of cast iron . Cast iron is an alloy of iron with 2–4% carbon. Silicon is also an important component of cast iron. A wide variety of very useful products can be cast from cast iron, such as manhole covers, pipeline fittings, engine cylinder blocks, etc.
Bronze is an alloy of copper, usually with tin as the main alloying component, and also with aluminum, silicon, beryllium, lead and other elements, excluding zinc. Tin bronzes were known and widely used in ancient times. Most antique bronzes contain 75-90% copper and 25-10% tin, which makes them look similar to gold, but they are more refractory. This is a very durable alloy. Weapons were made from it until they learned how to produce iron alloys. An entire era in human history is associated with the use of bronze: the Bronze Age.
Brass is an alloy of copper with Zn, Al, Mg. These are non-ferrous alloys with a low melting point and are easy to process: cut, weld and solder.
Cupronickel is an alloy of copper and nickel, sometimes with the addition of iron and manganese. In terms of external characteristics, cupronickel is similar to silver, but has greater mechanical strength. The alloy is widely used for making tableware and inexpensive jewelry. Most modern silver-colored coins are made from cupronickel (usually 75% copper and 25% nickel with minor additions of manganese).
Duralumin , or duralumin, is an aluminum-based alloy with the addition of alloying elements - copper, manganese, magnesium and iron. It is characterized by its steel strength and resistance to possible overloads. It is the main structural material in aviation and astronautics.
General methods for obtaining metals
The significant chemical activity of metals (interaction with atmospheric oxygen, other non-metals, water, salt solutions, acids) leads to the fact that in the earth’s crust they are found mainly in the form of compounds: oxides, sulfides, sulfates, chlorides, carbonates, etc. In free form, there are metals located in the voltage series to the right of hydrogen (Ag, Hg, Pt, Au, Cu), although much more often copper and mercury can be found in nature in the form of compounds.
Minerals and ferrous rocks containing metals and their compounds, from which the isolation of pure metals is technically possible and economically feasible, are called ores
.
Obtaining metals from ores is the task of metallurgy.
Metallurgy
is both the science of industrial methods for obtaining metals from ores and a branch of industry.
Any metallurgical process is a process of reduction of metal ions using various reducing agents. Its essence can be expressed as follows:
M n+ + ne−→M
To implement this process, it is necessary to take into account the activity of the metal, select a reducing agent, consider technological feasibility, economic and environmental factors.
In accordance with this, there are the following methods for obtaining metals:
• pyrometallurgical;
• hydrometallurgical;
• electrometallurgical.
Pyrometallurgy
Pyrometallurgy is the reduction of metals from ores at high temperatures using carbon, carbon monoxide (II), hydrogen, metals - aluminum, magnesium.
For example, tin is recovered from cassiterite SnO2, and copper from cuprite Cu2O
calcination with coal (coke):
SnO2+ 2С = Sn + 2СО ↑; Cu2O + C = 2Cu+ CO ↑
Sulfide ores are pre-roasted in the presence of air, and then the resulting oxide is reduced with coal:
2ZnS + 302 = 2ZnО + 2SO2 ↑; ZnO + C = Zn + CO ↑ sphalerite (zinc blende)
Metals are also isolated from carbonate ores by calcination with coal, since carbonates decompose when heated, turning into oxides, and the latter are reduced by coal:
FeСO3 = FeО + СO2 ↑ ; FeO + C = Fe + CO ↑ siderite (spar iron ore)
By reduction with coal one can obtain Fe, Cu, Zn, Cd, Ge, Sn, Pb and other metals that do not form strong carbides (compounds with carbon).
Hydrogen or active metals can be used as a reducing agent:
1) MoO3 + 3H2 = Mo + 3H2O (hydrothermy)
The advantages of this method include obtaining very pure metal.
2) TiO2+ 2Mg = Ti + 2MgO (magnesium thermia)
3МnO2 + 4Аl = 3Мn + 2Аl2O3 (aluminothermy)
Aluminum is most often used in metallothermy, the heat of oxide formation
which is very high (2A1 + 1.5 O2 = Al2O3 + 1676 kJ/mol). The electrochemical voltage series of metals cannot be used to determine the possibility of reactions occurring in the reduction of metals from their oxides. The possibility of this process can be approximately determined by calculating the thermal effect of the reaction (Q), knowing the values of the heats of formation of oxides:
Q= Σ Q1 - Σ Q 2,
where Q1 is the heat of formation of the product, Q2 is the heat of formation of the starting substance.
Blast furnace process (iron production):
C + O2 = CO2, CO2 + C ↔ 2CO 3Fe2O3 + CO = 2(Fe2Fe32)O4+ CO2 (Fe2Fe32)O4+ CO= 3FeO + CO2 FeO + CO= Fe + CO2 (cast iron contains up to 6.67% carbon in the form of graphite grains and cementite Fe3C);
Steelmaking
(0.2-2.06% carbon) is carried out in special furnaces (converter, open-hearth, electric), differing in the heating method.
Blowing air enriched with oxygen leads to the burning out of excess carbon, as well as sulfur, phosphorus and silicon in the form of oxides from the cast iron.
In this case, the oxides are either captured in the form of waste gases (CO2, SO2) or are bound into an easily separated slag - a mixture of Ca3(PO4)2 and CaSiO3. To produce special steels, alloying additives of other metals are introduced into the furnace.
Hydrometallurgy
Hydrometallurgy is the reduction of metals from their salts in solution.
The process takes place in two stages: 1) the natural compound is dissolved in a suitable reagent to obtain a solution of a salt of this metal; 2) from the resulting solution, this metal is displaced by a more active one or reduced by electrolysis. For example, to obtain copper from ore containing copper oxide CuO, it is treated with dilute sulfuric acid:
CuO + H2SO4 = CuSO4 + H2
Copper is then either removed from the salt solution by electrolysis or displaced from the sulfate by iron:
СuSO4. + Fe = Cu + FeSO4
In this way, silver, zinc, molybdenum, gold, and uranium are obtained.
Internal structure and physical properties of metals
Metals are simple substances whose atoms can only give up electrons. This feature of metals is due to the fact that at the outer level of these atoms there are few electrons (most often from 1 to 3) or the outer electrons are located far from the nucleus. The fewer electrons at the outer level of the atom and the further they are located from the nucleus, the more active the metal (the more pronounced its metallic properties).
Task 8.1. Which metal is more active:
Name the chemical elements A, B, C, D.
Metals and non-metals in Mendeleev's Periodic Table of Chemical Elements (PSM) are separated by a line drawn from boron to astatine. Above this line in the main subgroups are non-metals (see lesson 3). The remaining chemical elements are metals.
Task 8.2. Which of the following elements are metals: silicon, lead, antimony, arsenic, selenium, chromium, polonium?
Question. How can we explain the fact that silicon is a non-metal, and lead is a metal, although they have the same number of outer electrons?
An essential feature of metal atoms is their large radius and the presence of valence electrons weakly bound to the nucleus. For such atoms, the ionization energy* is small.
* IONIZATION ENERGY is equal to the work spent on removing one external electron from an atom (to ionize an atom) that is in the ground energy state.
Some of the valence electrons of metals, breaking away from atoms, become “free”. “Free” electrons easily move between atoms and metal ions in the crystal, forming an “electron gas” (Fig. 28).
At a subsequent moment in time, any of the “free” electrons can be attracted by any cation, and any metal atom can give up an electron and turn into an ion (these processes are shown in Fig. 28 by dotted lines).
Thus, the internal structure of a metal is similar to a layer cake, where positively charged “layers” of metal atoms and ions alternate with electronic “layers” and are attracted to them. The best model of the internal structure of a metal is a stack of glass plates moistened with water: it is very difficult to tear one plate from another (strong metals), and it is very easy to move one plate relative to another (ductile metals) (Fig. 29).
Task 8.3. Make such a “model” of the metal and verify these properties.
A chemical bond carried out by “free” electrons is called a metallic bond .
“Free” electrons also provide such physical properties of metals as electrical and thermal conductivity, plasticity (malleability), as well as metallic luster.
Task 8.4. Find metal objects around the house.
By completing this task, you can easily find metal utensils in the kitchen: pots, pans, forks, spoons. Machine tools, airplanes, cars, diesel locomotives, and tools are made from metals and their alloys. Modern civilization is impossible without metals, since electrical wires are also made of metals - Cu and Al. Only metals are suitable for making antennas for radio and television receivers; the best mirrors are made from metals. In this case, not pure metals are often used, but their mixtures (solid solutions) - ALLOYS.
Hydrometallurgy
Hydrometallurgy is the reduction of metals from their salts in solution.
The process takes place in two stages: 1) the natural compound is dissolved in a suitable reagent to obtain a solution of a salt of this metal; 2) from the resulting solution, this metal is displaced by a more active one or reduced by electrolysis. For example, to obtain copper from ore containing copper oxide CuO, it is treated with dilute sulfuric acid:
CuO + H2SO4 = CuSO4 + H2
Copper is then either removed from the salt solution by electrolysis or displaced from the sulfate by iron:
СuSO4. + Fe = Cu + FeSO4
In this way, silver, zinc, molybdenum, gold, and uranium are obtained.
conclusions
Metals are simple substances that are always reducing agents. The reduction activity of the metal decreases in the voltage series from lithium to gold. By the position of the metal in the stress series, you can determine how the metal reacts with acid solutions, with water, with salt solutions.
Lesson 9. Alkali and alkaline earth metals →
← Lesson 7. The concept of redox reactions