Chemical properties of alkali metals: interaction, preparation
Alkali metals are in the first group of the periodic table. The atoms of these elements contain one electron in their outermost energy level. It is located at a great distance from the core. Like all metals, they are reducing agents and easily give up electrons. The characteristic oxidation state is +1. In the group from top to bottom there is an increase in metallic properties. Due to the increasing ionization energy, the ability to donate electrons, and therefore electronegativity, increases from bottom to top.
Francium is the most active metal because it has an electron at the farthest distance from the nucleus. Accordingly, its ability to recover is the highest.
- Under normal conditions, alkali metals react with oxygen.
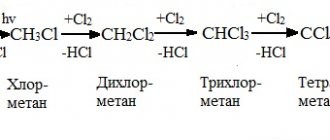
They are very active in such reactions, so they are stored under a layer of petroleum jelly. The reaction products can be oxides or peroxides. 4Li + O2 → 2Li2O 2Na + O2 → Na2O2 - Alkali metals react with water under normal conditions.
Hydrogen is displaced from the water, and a soluble base, an alkali, is formed as a reaction product. To recognize alkali, you can use an indicator - phenolphthalein. When added to a solution, it turns crimson. Reactions with water are very violent, lithium “explodes” in water, sodium “boils”. In this case, hydrogen is released in the form of white vapor in clouds. 2Na + 2H2O → 2NaOH + H2↑ 2Li + 2H2O → 2LiOH + H2↑ - React with halogens to form halides.
2K + Cl2 → 2KCl 2Na + Cl2 → 2NaCl - Characteristically, it reacts with hydrogen when heated; the reaction product is hydrides.
For example, the product of the reaction of potassium and hydrogen will be potassium hydride. H2↑ + 2K → 2KH - When heated, they form sulfides with sulfur.
It is a solid, colorless substance, soluble in water. 2Na + S → Na2S - When heated, a reaction occurs with phosphorus, phosphides being the product.
3K + P → K3P - Lithium and sodium can react with carbon when heated.
As a result, carbides are formed. The remaining alkali metals do not enter into these reactions. 2Li + 2C → Li2C2 - Under normal conditions, only lithium reacts with nitrogen; with other alkali metals, reaction is possible only when heated.
6Li + N2 → 2Li3N - They interact with alcohols to form alcoholates.
2C2H5OH + 2Na = 2C2H5ONa + H2↑Many alkali metals are capable of reacting with dilute acids to form hydrogen. However, the reaction proceeds in stages, i.e. First, the metal reacts with water to form an alkali, and then the alkali is neutralized with an acid. Interaction with acids is accompanied by an explosion and therefore such reactions are not carried out in practice.
Receipt
- The main method for obtaining alkali metals is the electrolysis of halide melts. In this case, chlorides that are part of natural minerals are most often used.
- Other methods for obtaining alkali metals can be obtained from their oxides and salts.
For example, sodium can be obtained by calcining soda with coal.Na2CO3 + 2C → 2Na + 3CO↑
Lithium is obtained from its oxide by raising the temperature to 300°C.
2Li2O + Si + 2CaO → 4 Li + Ca2SiO4
Reserves, production
Global quantities of copper ore are estimated at one billion tonnes (explored). The presence of half is confirmed. Scientists believe that the earth's crust hides another three billion tons of copper ore.
Native copper
Countries on all continents have rich reserves:
- America – Chile, Canada, USA.
- Asia – Kazakhstan, Iran.
- Africa – South Africa, Zambia, Zaire.
Russia accounts for 3% of world reserves. The deposits are concentrated in the Urals. The main producer is the Norilsk Nickel concern.
Ore is mined by open or closed methods, depending on the depth of occurrence.
The annual global ore production is 15-20 million tons.
Chemical properties of alkaline earth metals: interaction, preparation
The main subgroup of the second group of the periodic system of chemical elements is formed by metals, which are called alkaline earth metals.
They are so named because the hydrates of their oxides (“earths”), like the hydrates of alkali metal oxides, are alkalis.
The outer electron layer of their atoms consists of two electrons. By giving them away, the atoms of these metals turn into ions that carry two units of positive charge. In all their compounds, the metals of the beryllium subgroup are positively divalent. In the periodic table they are adjacent to the alkali metals. Therefore, these elements exhibit high chemical activity, second only to alkali metals. The properties of the metal increase with increasing atomic number.
- They react with oxygen, the reaction product becomes oxides, except for barium, it forms peroxide BaO2. Beryllium and magnesium interact with oxygen only at very high temperatures, since they are covered with a thin protective oxide film.
2Ca + O2 → 2CaOIn the above reaction, a piece of calcium burns to produce white smoke when heated. It is formed by the finest solid particles of calcium oxide.
- Like alkali metals, they react with water, but less actively. As a result, an oxide hydrate is formed and hydrogen is displaced.
Ca + 2H2O → 2Ca(OH)2 + H2↑Phenolphthalein turns crimson in the resulting solution. This example justifies the expected similarity in the chemical properties of alkaline earth and alkali metals: both react with water to release hydrogen. Alkaline earth metal oxide hydrates, like alkalis, are alkalis, meaning they are soluble in water.
- All metals except beryllium react with halogens.
Beryllium reacts with halogens only at elevated temperatures. The reaction product is halides. Ca + Cl2 → CaCl2 - When heated, all alkaline earth metals, except beryllium, react with hydrogen.
As a result, hydrides are formed. Ca + H2 → CaH2 - React with sulfur, resulting in the formation of sulfides.
Ca + S → CaS - Reacts with nitrogen when heated, with the exception of magnesium. It reacts with nitrogen under normal conditions. The reaction product is nitrides.
3Be + N2 → Be3N2 3Mg + N2 → Mg3N2
- They can react with acids, resulting in the formation of salts of the corresponding acid and hydrogen.
Be + H2SO4 (dil.) → BeSO4 + H2↑
Receipt
The main methods for producing metals of the second group of the main subgroup are electrolysis of melts, aluminothermy and the displacement of other more active metals from their salts.
CaO + Al → Al2O3 + Ca
MgBr2 + Ca → CaBr2 + Mg
Story
Copper is one of the first metals that humanity dealt with. This was facilitated by the advantages: high prevalence, availability, relatively low melting point.
People appreciated the benefits of copper eight thousand years ago.
The Copper Age began immediately after the Stone Age:
- Copper artifacts dug up in the territory of modern Turkey are recognized as the oldest. These are beads and decorative overlays.
- Cutting tools and utensils were made from metal.
- The history of the discovery of copper mines in Rus' begins in the Urals two thousand years before the new era. Then there were the Caucasus, Altai, Siberia.
- Industrial processing using bronze began in the 14th century. Cannons and bells were cast from the alloy.
The Tsar Bell and the Tsar Cannon were cast from bronze.
It is assumed that the metal is named after the island of Cyprus. Copper deposits were discovered here back in the 3rd century BC, and the population mastered copper smelting.
M. Vasmer’s “Etymological Dictionary of the Russian Language” links the origin of the Russian term copper with the ancient German root smid – blacksmith, metal.
Chemical properties of aluminum
Aluminum is in the third group of the periodic table of elements. The charge of the nucleus of an aluminum atom is +13, there are three electrons in the outer electron layer.
Based on the structure of the atoms and their position in the periodic table, it can be assumed that the metallic properties of the elements of the third group should be less pronounced than those of the elements of the second group. This is true.
During chemical reactions, an aluminum atom gives up three electrons from its outer layer, turning into a three-charged positive ion Al3+. Therefore, in all its stable compounds, aluminum is positively trivalent. Its compounds exhibit amphoteric properties.
Aluminum is a reactive metal and acts as a reducing agent. However, its activity is reduced by the oxide film that forms on its surface. Therefore, in many reactions the film is first removed, and then the reaction with substances occurs. Let's look at the chemical properties of aluminum using specific examples.
- Aluminum combines with oxygen in the air both when heated and at ordinary temperatures. A thin, dense film of aluminum oxide quickly forms on its surface. It is difficult to permeate gases and protects the metal from further oxidation.
In a crushed state and at elevated temperatures, aluminum reacts violently with oxygen, releasing a large amount of heat. As a result, aluminum oxide is formed.4Al + 3O2 → 2Al2O3
- Reactions with many nonmetals occur when heated.
2Al + 3S → Al2S3 Al + P → AlP 2Al + N2 → 2AlN 4Al + 3C → Al4C3 - It interacts with water to remove the oxide film.
The reaction proceeds vigorously, displacing hydrogen from water. 2Al + 6H2O → 2Al(OH)3↓ + 3H2 - Interaction with acids. Place aluminum shavings in a test tube containing hydrochloric or dilute sulfuric acid. Aluminum dissolves, displacing hydrogen from the acid and forming a salt.
2Al + 6HCl → 2AlCl3 + 3H2↑ 2Al +3H2SO4(dil.)→ Al2(SO4)3 + 3H2↑Does not react with concentrated nitric and sulfuric acid. Therefore, concentrated nitric acid is stored in aluminum containers and transported in aluminum tanks.
Reacts with dilute nitric acid to form
N2O, N2 or NH4NO3. 8Al + 30HNO3 → 8Al(NO3)3 + 3N2O + 15H2O
- Since aluminum has amphoteric properties, it is characterized by reactions with alkalis.
2Al + 2NaOH + 10H2O → 2Na[Al(H2O)2(OH)4] + 3H2↑ - Aluminum interacts with the oxides of most metals, displacing the less active metal.
This method is used in industry to obtain metals and is called aluminothermy. 2Al + Fe2O3 → 2Fe + Al2O3
Let's sum it up
Their chemical properties depend on the activity of metals. Simple substances - metals - are reducing agents in redox reactions. By the position of a metal in the electrochemical series, one can judge how actively it is capable of entering into chemical reactions (i.e., how strongly the metal exhibits reducing properties).
Finally, we’ll share a table that will help you remember what metals react with and prepare for your chemistry test.
How to smelt cuprum
- pyrometallurgical (with its help, 90% of the metal is produced);
- hydrometallurgical, the remaining 10%.
Hydrometallurgy consists of a single step: treating (usually low-grade) ore with dilute sulfuric acid and then separating copper metal from the solution. In this case, all associated substances from the ore simply disappear.
Pyrometallurgy is more complicated, there are several stages:
- Enrichment by flotation and oxidative roasting.
- Melting for matte at temperatures up to 1500 degrees. Here, crude metal is already isolated, as well as accompanying silver, gold, and nickel.
- Fire refining - purification of the resulting metal from impurities to 99.5% purity.
- Electrolytic refining, bringing purity to 99.95%.
Alloys
The range of copper alloys with other components includes dozens of items.
Copper alloys and their applications
They are used more often than pure metal because they reduce the disadvantages inherent in pure metal. That is, they make the product stronger, more stable, and cheaper.
Copper connections are divided into two groups:
- Bronze - with tin.
- Brass – with zinc.
In addition to these main alloying components, the compound contains aluminum, nickel, bismuth, titanium, silver, gold, and non-metallic elements.
From Turkey to Egypt
The history of the discovery of metal has been lost for centuries. Who first discovered the metal, who guessed to “tap” the nugget so that it would turn into a blade or other tool is unknown. Moreover, no one knows who came up with the idea of “welding” the nugget and pouring the liquid metal into the mold.
The next mystery is who was the first to smelt ore into metal. But it is known that archaeologists found the most ancient finds of copper products and smelting slag in modern Turkey. They are incredibly ancient - they are up to 10,000 years old.
Why copper?
The tribes who lived in Europe in ancient times called copper and any metals “mida”. The word “copper” is also found in ancient Russian texts. Scholars consider the word to be related to the Old German "smid" (blacksmith); or a derivative of Media, a country on the territory of present-day Iran.
In Latin, copper is called cuprum (aes cuprium), from the island of Cyprus. There was a rich metal deposit there. Pliny writes:
“...It is known how long the Roman people used only copper coins. Antiquity itself testifies to the importance of this metal.”
For a long time, the main common coin of the Roman Empire was called ass (aes).
Now “copper” and “cuprum” peacefully share their belonging to the non-ferrous metal.
Areas of application
The properties of the metal determine its use in various fields. The main consumer is the industrial complex.
Industry
Metal and alloys are used by the following industries:
- Electrical engineering, radio electronics. Cables (power, others), wires. Winding in transformers. Heat exchange devices (heating radiators, air conditioners, computer coolers, laptop heat pipes).
- Instrumentation, mechanical engineering. Parts and machine components are made from alloys of copper with zinc, tin, and aluminum. Without it, it is impossible to create galvanic cells and batteries.
- Pipes. For transporting steam, water, gas. In the energy sector, shipbuilding, for domestic needs.
Copper heat pipe cooling system in a laptop
In Japan, copper pipelines are recognized as earthquake-resistant, which is vital for this country.
Copper pipes
Construction
Roofs made of copper sheets are environmentally friendly; they do not need to be painted, since moisture and weather disasters are not scary. Service life – up to 100 years.
Medicine
The characteristics of the metal as an antiseptic and astringent are in demand in medicine.
It is a component of eye drops and mixtures for the treatment of burns.
Copper door handles and other surfaces are an attribute of medical institutions.
Copper compounds suppress the swine flu virus.
Jewelry
Jewelers use copper-based alloys.
Copper ring
Red or rose gold is a conglomerate of the noble metal with copper.
Its amount in the composition determines the final shade:
- 25% – pink;
- 50% – red.
These types of gold are the most beloved by jewelers. Copper makes products stronger, while simultaneously reducing the cost.
The second popular jewelry alloy is cupronickel (copper + nickel).
Other industries
- Copper oxide is the basis of cuprate, used in superconductors.
- Brass is used to make cartridges for rifles and artillery.
- Nickel silver is used to mint coins, create interior decorations, and cutlery.
- Copper is involved in the synthesis of chlorophyll. It is always added to mineral fertilizers for plants.
Meaning for humans
Copper is inherent in the human body initially:
- Participates in the formation of red blood cells, collagen, elastin.
- Activates the endocrine system, slows down the aging of the body.
- Its deficiency is fraught with a slowdown in protein metabolism. This leads to pathologies in the development of the skeleton and blood composition.
It is found in many foods. Beef liver, oysters, sesame seeds, cocoa powder, black pepper, and buckwheat are rich in copper. And also nuts (hazelnuts, walnuts, cashews, peanuts, almonds).
Copper in nature
In nature, two manifestations of the element have been identified - nuggets and components of compounds with other elements.
Copper nugget
Most often these are compounds: oxides, sulfides, bicarbonates. The most common raw material is copper pyrite.
Copper gives deep blue, cyan, greenish shades to malachite, turquoise, chrysocolla, and other minerals of the jewelry and decorative segment.