Zinc is a typical representative of the group of metallic elements and has the full range of their characteristics: metallic luster, ductility, electrical and thermal conductivity. However, the chemical properties of zinc differ somewhat from the basic reactions inherent in most metals. An element can behave like a nonmetal under certain conditions, for example, react with alkalis. This phenomenon is called amphotericity. In our article we will study the physical properties of zinc, and also consider typical reactions characteristic of the metal and its compounds.
Position of the element in the periodic table and distribution in nature
The metal is located in a secondary subgroup of the second group of the periodic table. In addition to zinc, it contains cadmium and mercury. Zinc belongs to the d-elements and is in the fourth period. In chemical reactions, its atoms always give up electrons of the last energy level, therefore, in such compounds of the element as oxide, intermediate salts and hydroxide, the metal exhibits an oxidation state of +2. The structure of the atom explains all the physical and chemical properties of zinc and its compounds. The total metal content in the soil is approximately 0.01 wt. %. It is found in minerals such as galmea and zinc blende. Since the zinc content in them is low, the rocks are first subjected to enrichment, which is carried out in shaft furnaces. Most zinc-containing minerals are sulfides, carbonates and sulfates. These are zinc salts, the chemical properties of which underlie their processing processes, such as roasting.
Metal production
The severe oxidation reaction of zinc carbonate or sulfide produces its oxide. The process takes place in a fluidized bed. This is a special method based on close contact of finely ground mineral and a stream of hot air moving at high speed. Next, zinc oxide ZnO is reduced with coke and the resulting metal vapors are removed from the reaction sphere. Another method of producing metal, based on the chemical properties of zinc and its compounds, is electrolysis of a solution of zinc sulfate. It is a redox reaction that occurs under the influence of electric current. High purity metal is deposited on the electrode.
Historical reference
The name “zinc” itself was first mentioned in the book “Liber Mineralium” by Paracelsus. According to some sources, it meant “prong.” The alloy of zinc with copper or brass has been known for a long time. It was used in Ancient Greece, India and Ancient Egypt, and later the material became known in China.
The metal was obtained in its pure form only in the first half of the 18th century in 1738 in Great Britain using the distillation method. Its discoverer was William Champion. Industrial production began 5 years later, and in 1746 in Germany, the chemist Andreas Sigismund Marggraff developed and described in detail his own method for producing zinc . He proposed using the method of calcining a mixture of metal oxide and coal in fireproof clay retorts without air access. Subsequent condensation of the vapors had to take place in the refrigerator. Due to his detailed description and painstaking development, Marggraf is often called the discoverer of the substance.
Read also: How to change the rotation on a commutator motor
At the beginning of the 19th century, a method was found for isolating metal by rolling at 100 C o -150 C o . At the beginning of the next century, they learned to extract zinc using the electrolytic method. In Russia, the first metal was produced only in 1905.
Physical characteristics
A bluish-silver, brittle metal under normal conditions. In the temperature range from 100° to 150°, zinc becomes flexible and can be rolled into sheets. When heated above 200°, the metal becomes unusually brittle. Under the influence of atmospheric oxygen, pieces of zinc are covered with a thin layer of oxide, and upon further oxidation it turns into hydroxycarbonate, which plays the role of a protector and prevents further interaction of the metal with atmospheric oxygen. The physical and chemical properties of zinc are interrelated. Let's consider this using the example of the interaction of a metal with water and oxygen.
In food
The element is found in meat, cheese, sesame seeds, oysters, chocolate, legumes, oatmeal, sunflower and pumpkin seeds, and is often present in mineral water. The highest percentage of zinc is found in the following products (per 100 grams):
- Oysters (up to 40 mg), anchovies (1.72 mg), octopus (1.68 mg), carp (1.48 mg), caviar (up to 1 mg), herring (about 1 mg).
- Pumpkin seeds (10 mg), sesame seeds (7 mg), sunflower seeds (5.3 mg), peanuts (4 mg), walnuts (3 mg), almonds (3 mg).
- Beef (up to 8.4 mg), lamb (up to 6 mg), beef liver (4 mg), pork (up to 3.5 mg), chicken (up to 3.5 mg).
- Cocoa powder without sugar and sweeteners (6.81 mg), pure dark chocolate (2.3 mg), chocolate candies (up to 2 mg depending on the amount and type of chocolate).
- Lentils (4.78 mg), oats (3.97 mg), wheat (3.46 mg), soybeans (3 mg), rye (2.65 mg), bread (up to 1.5 mg), green peas (1.24 mg), peas (1.2 mg), bamboo shoots (1.1 mg), rice (1 mg), cereal cookies (up to 1 mg).
- Hard cheese (up to 4 mg).
Redox reactions involving zinc
Since the element comes before hydrogen in the activity series of metals, it is able to displace it from acid molecules.
The reaction products between zinc and acids will depend on two factors:
- type of acid
- its concentration
Dilute sulfuric acid, which does not exhibit pronounced oxidizing properties, reacts with the metal according to the following scheme:
H₂SO₄ + Zn = ZnSO₄ + H₂↑
Reactions of the element with phosphoric and dilute sulfuric acids proceed in the same way. The chemical properties and reactions of zinc with nitrate acid have their own characteristics. A dilute solution of nitric acid of average concentration and zinc interact with each other to form nitrogen oxide (II), water and an average salt - zinc nitrate. Concentrated nitrate acid reacts with the metal in such a way that nitric oxide (IV), average salt and water can be detected in the products.
A very dilute solution of nitric acid and zinc as a reducing agent react to form zinc nitrate, water, and several possible products: ammonia, free nitrogen, or nitric oxide.
Features of smelting
The temperature required to melt zinc should be less than 419 C o, but not more than 480 C o. Otherwise, metal waste will increase and wear on the walls of the bath, which is usually made from iron, will increase. In the molten state, no more than 0.05% iron admixture is allowed, otherwise the temperature required for melting will begin to rise. If the percentage of iron content exceeds 0.2%, zinc cannot be rolled.
Zinc is obtained from polymetallic ores, which can contain up to 4% of the element . If the ores have been enriched by selective flotation, up to 60% of zinc concentrates can be obtained from them, the rest will be occupied by concentrates of other metals. Zinc concentrates are fired in furnaces in a fluidized bed, after which zinc sulfide turns into oxide and sulfur dioxide is released. The latter is consumed: sulfuric acid is obtained from it.
To convert zinc oxide into the metal itself, two methods are used.
- Distillation or pyrometallurgical. The concentrate is fired, then sintered to impart gas permeability and granularity and reduced with coke or coal at temperatures of 1200-1300 C o. During the reaction, metal vapors are formed, which are condensed and poured into molds. The purity of zinc reaches 98.7%, after which it can be increased to 99.995% using rectification, but the latter method is quite expensive and complex.
- Electrolytic or hydrometallurgical. The fired concentrates are treated with sulfuric acid, the solution is cleaned of impurities using zinc dust and subjected to electrolysis in bathtubs lined with lead or vinyl plastic. Zinc is deposited on aluminum cathodes, from where it is collected and melted in induction furnaces. The purity of the metal obtained by this method reaches 99.95%.
Chemical properties of zinc
The reaction equations for the interaction of a metal with alkali solutions confirm its amphoteric properties. Complex salts - tetrahydroxycinates and hydrogen - are found in the products.
Zn + 2NaOH + 2H2O = Na2 [Zn(OH)4] + H2
By fusing solid alkali and metal, they obtain salts of another type - zincates. A by-product of such a process will also be hydrogen gas.
Zn + 2KOH = K2ZnO2 + H2
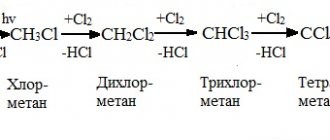
The metal actively interacts with halogens, for example, chlorine, bromine or iodine, as well as with nitrogen, sulfur and carbon. As a result, intermediate salts are formed - nitrides, sulfides or carbides.
In the activity series of metals, zinc is placed before hydrogen and is therefore an active metal. However, it is inferior in its properties to alkali and alkaline earth metals.
Use of zinc in galvanic cells
The chemical properties of zinc underlie the operating principle of various types of galvanic devices. The manganese-zinc element is the most common in technology. It works by undergoing a redox reaction between the metal and manganese dioxide. Both electrodes are made from them and placed inside the device. The active substance - ammonium chloride - has the form of a paste, or porous plates inserted between the cathode and anode are impregnated with it. The zinc air element is represented by a negative zinc electrode - cathode. The anode is a carbon-graphite rod filled with air. Solutions of ammonium chloride or sodium hydroxide are used as an electrolyte.
Zinc oxide
A white porous powder that turns yellow when heated and returns to its original color when cooled is a metal oxide. The chemical properties of zinc oxide and the reaction equations for its interaction with acids and alkalis confirm the amphoteric nature of the compound. Thus, the substance cannot react with water, but interacts with both acids and alkalis. The reaction products will be medium salts (in case of interaction with acids) or complex compounds - tetrahydroxocinates.
Zinc oxide is used in the production of white paint, which is called zinc white. In dermatology, the substance is included in ointments, powders and pastes that have an anti-inflammatory and drying effect on the skin. Most of the zinc oxide produced is used as a filler for rubber. Continuing to study the chemical properties of zinc and its compounds, let's consider Zn(OH)2 hydroxide.
Amphoteric nature of zinc hydroxide
The white precipitate that precipitates under the action of alkali on solutions of metal salts is the base of zinc. The compound dissolves quickly when exposed to acids or alkalis. The first type of reaction ends with the formation of medium salts, the second - zincates. Complex salts—hydroxycinates—are isolated in solid form. A special feature of zinc hydroxide is its ability to dissolve in an aqueous solution of ammonia to form tetraamminium zinc hydroxide and water. Zinc base is a weak electrolyte, therefore both its average salts and zincates in aqueous solutions are hydrolyzable, that is, their ions react with water and form zinc hydroxide molecules. Solutions of metal salts such as chloride or nitrate will be acidic due to the accumulation of excess hydrogen ions.
Characteristics of zinc sulfate
The chemical properties of zinc that we examined earlier, in particular, its reactions with dilute sulfate acid, confirm the formation of an average salt - zinc sulfate. These are colorless crystals, which, when heated to 600° and above, can produce oxosulfates and sulfur trioxide. With further heating, zinc sulfate is converted to zinc oxide. The salt is soluble in water and glycerin. The substance is isolated from solution at temperatures up to 39°C in the form of crystalline hydrate, the formula of which is ZnSO4 × 7H2O. In this form it is called zinc sulfate.
In the temperature range 39°-70°, a hexahydrate salt is obtained, and above 70° only one molecule of water remains in the crystalline hydrate. The physicochemical properties of zinc sulfate make it possible to use it as a bleach in paper production, as a mineral fertilizer in crop production, and as a fertilizer in the diet of domestic animals and poultry. In the textile industry, the compound is used in the production of viscose fabric and in the dyeing of chintz.
Zinc sulfate is also included in the electrolyte solution used in the process of galvanic coating of iron or steel products with a layer of zinc using the diffuse method or hot-dip galvanizing method. A layer of zinc protects such structures from corrosion for a long time. Considering the chemical properties of zinc, it should be noted that in conditions of high salinity of water, significant fluctuations in temperature and air humidity, galvanizing does not give the desired effect. Therefore, metal alloys with copper, magnesium and aluminum are widely used in industry.
Production
As stated, zinc is not found in nature in its pure form. It is mainly obtained from polymer ores. In these ores, zinc is present in the form of sulfide. It always comes with the accompanying metals listed above.
The selective flotation beneficiation process produces zinc concentrate. In parallel with this process, other substance concentrates are released from polymetallic ores. For example, lead and copper.
The resulting zinc concentrates are fired in a furnace. As a result of high temperatures, zinc changes from the sulfide state to the oxide state. During the production process, sulfur dioxide is released, which is used to produce sulfuric acid. Pure zinc is obtained from zinc oxide by two methods: pyrometallurgical and electrolytic.
- The pyrometallurgical method has a very long history. The concentrate is fired and subjected to a sintering process. The zinc is then reduced using coal or coke. The zinc obtained by this method is brought to a pure state by settling.
- In the electrolytic method, zinc concentrate is treated with sulfuric acid. The result is a solution that is subjected to the process of electrolysis. Here the zinc is deposited and melted in special furnaces.
Read also: What is tensile strength?
Application of alloys containing zinc
Transporting many chemicals, such as ammonia, through pipelines requires special requirements for the composition of the metal from which the pipes are made. They are made on the basis of alloys of iron with magnesium, aluminum and zinc and have high anti-corrosion resistance to aggressive chemical environments. In addition, zinc improves the mechanical properties of alloys and neutralizes the harmful effects of impurities such as nickel and copper. Copper and zinc alloys are widely used in industrial electrolysis processes. Tankers are used to transport petroleum products. They are built from aluminum alloys containing, in addition to magnesium, chromium and manganese, a large proportion of zinc. Materials of this composition not only have high anti-corrosion properties and increased strength, but also cryogenic resistance.
Mixtures and alloys
To enhance strength and increase the melting point, the metal is mixed with copper, aluminum, tin, magnesium and lead.
The most famous and sought after alloy is brass. This is a mixture of copper with the addition of zinc, sometimes tin, nickel, manganese, iron, and lead are also found. The density of brass reaches 8700 kg/m3 . The temperature required for melting is kept at around 880 C o - 950 C o: the higher the zinc content in it, the lower it is. The alloy perfectly resists unfavorable external environments, although it turns black in air if not varnished, it is perfectly polished and welded by resistance welding.
There are two types of brass:
- Alpha brass: more ductile, bends well in any condition, but wears out more.
- Alpha+beta brass: deforms only when heated, but is more wear-resistant. Often alloyed with magnesium, aluminum, lead and iron. This increases strength, but reduces ductility.
Zamak or Zamac alloy is composed of zinc, aluminum, copper and magnesium . The name itself is formed from the first letters of the Latin names: Zink - Aluminum - Magnesium - Kupfer / Cuprum (Zinc-Aluminum-Magnesium-Copper). In the USSR, the alloy was known as TsAM: Zinc-Aluminum-Copper. Actively used in injection molding, melting begins at low temperatures (381 C o - 387 C o) and has a low coefficient of friction (0.07). It has increased strength, which makes it possible to produce products of complex shapes that are not afraid of breaking: door handles, golf clubs, firearms, construction fittings, fasteners of various types and fishing tackle.
Read also: How to cut laminate flooring with a jigsaw without chipping
A small percentage of zinc (no more than 0.01%) is contained in hart alloys used in printing for casting typographic fonts and rulers, printing forms and typesetting. These are outdated mixtures, replaced by pure zinc with a small addition of impurities.
The low temperature required to melt zinc is often compensated for by alloys with other metals, but it also happens vice versa. If the temperature required to melt a “pure” metal is 419.5 C o , then the alloy with tin is reduced to 199 C o, and with tin and lead - to 150 C o. And although such alloys can be soldered and welded, most often mixtures with zinc are used only to seal existing defects due to their weak strength. For example, an alloy of tin, lead and zinc is recommended for use only on nickel-plated products.
Most often, zinc alloys are used to create carburetors, speedometer frames, radiator grilles, hydraulic brakes, pumps and decorative elements, parts for washing machines, mixers and kitchen equipment, watch cases, typewriters, cash registers and household appliances. These parts cannot be used in industrial production: when the temperature rises to 100 C, the strength of the product decreases by a third, and hardness by almost 40%. When the temperature drops to 0 C, zinc becomes too brittle, which can lead to breakage.
The role of zinc in the human body
The Zn content in cells is 0.0003%, so it is classified as a microelement. The chemical properties and reactions of zinc and its compounds play an important role in metabolism and maintaining a normal level of homeostasis, both at the level of the cell and the entire organism as a whole. Metal ions are part of important enzymes and other biologically active substances. For example, it is known that zinc has a serious effect on the formation and functions of the male reproductive system. It is part of the coenzyme of the hormone testosterone, which is responsible for the fertility of seminal fluid and the formation of secondary sexual characteristics. The non-protein part of another important hormone, insulin, produced by the beta cells of the islets of Langerhans in the pancreas, also contains a trace element. The immune status of the body is also directly related to the concentration of Zn+2 ions in cells, which are found in the thymus hormone - thymulin and thymopoietin. A high concentration of zinc is recorded in nuclear structures - chromosomes containing deoxyribonucleic acid and participating in the transmission of hereditary information of the cell.
In our article, we studied the chemical functions of zinc and its compounds, and also determined its role in the life of the human body.
A Brief History of the Element's Discovery
People knew what zinc was even before our era. After all, it was then that they learned to use alloys containing this metal. The Egyptians used ores containing copper and zinc, smelted them and obtained a very strong, oxidation-resistant material. Household items and dishes made from this material were found.
The name zinc appears in the writings of the physician Paracelsus in the 16th century AD. During the same period, the Chinese began to actively use the metal, casting coins from it. Gradually, knowledge about this substance and its good technical properties is spreading to Europe. Then in Germany and England they also learned what zinc is and where it can be used.
Brass was one of the first and most famous alloys, used since ancient times in Cyprus and later in Germany and other countries.
The name comes from the Latin zincum, but the etymology is not entirely clear. There are several versions.
- From the German zinke, which translates as "edge".
- From the Latin zincum, meaning "white coating".
- Persian "cheng", that is, stone.
- Old German zinco, which translates as “plaque”, “eyesore”.
The element received its current name only at the beginning of the 20th century. The importance of zinc ions in the human body also became known only relatively recently (20th century). Before this, no ailments were associated with this element.
However, it is known that already in ancient times, many peoples used soups made from young lamb meat as a means of recovery from illness and for a speedy recovery. Today we can say that the effect was achieved due to zinc ions, which this dish contains quite a lot. It helped restore blood circulation, relieve fatigue and activated brain activity.