In industry and everyday life, cast iron products are widely used. The metal is iron, which has 2 percent carbon integrated into its molecular structure. Today, many grades of metal are produced that have different fracture characteristics. About a hundred species.
Production requires a huge amount of thermal energy, since the melting point of cast iron is over one thousand degrees Celsius. Melting occurs at a temperature of 1150 - 1200 C°.
In addition to carbon, to obtain the required grade, silicon, sulfur, manganese, and phosphorus are added to the batches. Increased strength is achieved by incorporating alloying additives into the batches.
Material classification
This material is more brittle than steel . It can collapse without noticeable deformation. Carbon in the alloy takes the form of graphite and cementite, or each substance is presented separately. Varieties of cast iron appear in connection with their shape and quantity:
- White. All carbon is in the form of cementite. This color of the material is visible at the fracture. It can be described as brittle but hard. It is processed mainly to produce the malleable variety.
- Grey. Carbon in the form of a plastic form of graphite. It is characterized as soft, easy to process using low melting temperatures.
- Malleable. This type is named conventionally, since the material is not forged. This type is obtained as a result of prolonged firing of white, after which graphite is formed. The properties of the material are negatively affected by heating above 900 degrees, as well as by the cooling rate of graphite. As a result, the welding and processing process becomes difficult.
- Highly durable. It contains spherical graphite, which is formed through crystallization.
Cast iron and steel
Cast iron is an alloy of carbon and iron, it contains impurities of manganese, silicon, sulfur and phosphorus. Withstands low voltages and loads. One of its many advantages is its low cost for consumers. There are four types of cast iron:
- White - has high strength and poor ability to be processed with a knife. Types of alloy according to the increase in the amount of carbon in the composition: hypoeutectic, eutectic, hypereutectic. It was called white due to the fact that it has a white color in the fault. White cast iron also has a special structure of the metal mass and great wear resistance. Useful in making mechanical parts that will operate in a non-lubricated environment. It is used to make the following types of cast iron.
- Gray cast iron - contains carbon, silicon, manganese, phosphorus and some sulfur. It can be easily obtained and has poor mechanical properties. Used for the manufacture of parts that are not exposed to shock loads. There is a gray color in the fracture; the darker it is, the softer the material. The properties of gray cast iron depend on the temperature of the environment in which it is located and the amount of various impurities.
- Malleable cast iron is obtained from white cast iron as a result of simmering (prolonged heating and holding). The substance contains: carbon, silicon, manganese, phosphorus, and a small amount of sulfur. It is more durable and ductile, easier to process.
- Ductile iron is the strongest of all types of cast iron. Contains carbon, manganese, sulfur, phosphorus, silicon. Has high impact strength. This important metal is used to make pistons, crankshafts and pipes.
The melting points of steel and cast iron are different, as stated in the table above. Steel has higher strength and resistance to high temperatures than cast iron, temperatures differ by as much as 200 degrees. For cast iron, this number ranges from approximately 1100 to 1200 degrees, depending on the impurities it contains.
Differences between steel and cast iron
The difference in materials is expressed as follows:
- Cast iron is less hard and durable than steel.
- Steel is heavier and has a higher melting point.
- Since steel has a lower carbon content, it is easier to process (forging, cutting, welding, rolling). For this reason, cast iron products are made by casting.
- Cast iron products are porous (due to casting), so their thermal conductivity is lower.
- Artwork made of steel has a shine and shine, while art products made of cast iron are black and matte.
- Cast iron is the primary product of ferrous metallurgy, and steel is the final product.
- Steel is usually subjected to a hardening procedure.
- Cast iron products are produced through the casting process, while steel products are forged and welded.
Boiling and melting points of metals
The table shows the melting point of metals tmelt , their boiling point tk at atmospheric pressure, the density of metals ρ at 25°C and thermal conductivity λ at 27°C.
The melting point of metals, as well as their density and thermal conductivity are given in the table for the following metals: actinium Ac, silver Ag, aluminum Al, gold Au, barium Ba, beryllium Be, bismuth Bi, calcium Ca, cadmium Cd, cobalt Co, chromium Cr, cesium Cs, copper Cu, iron Fe, gallium Ga, hafnium Hf, mercury Hg, indium In, iridium Ir, potassium K, lithium Li, magnesium Mg, manganese Mn, molybdenum Mo, sodium Na, niobium Nb, nickel Ni, neptunium Np , osmium Os, protactinium Pa, lead Pb, palladium Pd, polonium Po, platinum Pt, plutonium Pu, radium Ra, rubidium Pb, rhenium Re, rhodium Rh, ruthenium Ru, antimony Sb, tin Sn, strontium Sr, tantalum Ta, technetium Tc, thorium Th, titanium Ti, thallium Tl, uranium U, vanadium V, tungsten W, zinc Zn, zirconium Zr.
According to the table, it can be seen that the melting point of metals varies over a wide range (from -38.83°C for mercury to 3422°C for tungsten). Metals such as lithium (18.05°C), cesium (28.44°C), rubidium (39.3°C) and other alkali metals have a low positive melting point.
The most refractory metals are the following: hafnium, iridium, molybdenum, niobium, osmium, rhenium, ruthenium, tantalum, technetium, tungsten. The melting point of these metals is above 2000°C.
Here are examples of the melting point of metals widely used in industry and everyday life:
- melting point of aluminum 660.32 °C;
- copper melting point 1084.62 °C;
- melting point of lead 327.46 °C;
- melting point of gold 1064.18 °C;
- melting point of tin 231.93 °C;
- the melting point of silver is 961.78 °C;
- The melting point of mercury is -38.83°C.
Rhenium Re has the maximum boiling point of the metals presented in the table - it is 5596°C. Also, metals belonging to the group with a high melting point have high boiling points.
The density of the metals in the table ranges from 0.534 to 22.59 g/cm 3 , that is, the lightest metal is lithium, and the heaviest metal is osmium. It should be noted that osmium has a density greater than that of uranium and even plutonium at room temperature.
The thermal conductivity of metals in the table varies from 6.3 to 427 W/(m deg), thus the worst conductor of heat is a metal such as neptunium, and the best heat-conducting metal is silver.
Cast iron: melting point
Advantages of the material
This material good casting properties , good fluidity, and a lower melting point compared to steel and ductile iron. These properties are taken into account when making the mold.
The gaseous flux is most often used for welding materials with brass. Copper-coated cast iron rods are also used, which improve the wettability of the edging with the deposited metal. Rods made of eutectic cast iron are used; its melting point is in the range of 1050 - 1200 degrees . Welding also occurs thanks to fluxes, which are used in the form of a paste. If special cast iron rods or L-62 brass are not available, then cracks in parts made of this material can be welded with wire made of electrolytic red copper.
Significantly above the melting point, cast iron overheats, which causes these suspended particles to dissolve, perhaps not completely, and this makes the formation of graphite more difficult. In some cases, it can occur when various substances are added to cast iron, which causes additional centers of graphite crystallization to appear.
Cast iron has better casting properties when compared to steel. Ease of use, as well as good fluidity and mold fillability, are ensured by a lower melting point and the final crystallization process at a constant temperature.
The above advantages of cast iron turn the material into a valuable structural material, which is widely used in machine parts when they are not subject to significant tensile and impact loads.
Melting point of semi-synthetic material
Semi-synthetic cast iron is melted by melting the charge, and the temperature range ranges from 1400-1450 degrees . After melting the charge, the cast iron is stored in the furnace crucible at slight overheating, not exceeding the melting point by one hundred degrees. What needs to be done to create a slag cover? When the charge gradually begins to melt, crushed glass or calcined quartz sand should be applied to the metal surface.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal applications, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field . The impact is approximately the same.
When heating occurs , the amplitude of thermal vibrations of molecules increases. Structural lattice defects appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting - from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory - from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Types of welding
Gas welding should be carried out by melting with a flame parts of the elements being connected and a rod of filler metal. This welding is used to connect metal parts, non-metallic elements and alloys that have different melting points, and the thickness should be no more than 30 mm . To arrange it, you do not need to resort to electricity.
Electric arc welding is widely used. Thanks to the electric arc, the melted metal, which combines various elements, interacts with the metal of the electrode, forming a strong seam. flux or inert gases are used for this . Electric arc welding uses various methods (manually, semi-automatically and automatically) to connect parts made of cast iron, structural steel, copper, aluminum and other alloys.
The melting point depends on the carbon contained in the material. The more it is, the lower the temperature, and the higher the fluidity when heated. From this we can conclude that the material is fluid, brittle, non-plastic and difficult to process. Its specific gravity is 6.9 G/cm3. The melting point is in the range of 1150-1250 degrees.
Stainless steel
Stainless steel is one of the many iron alloys found in steel. It contains Chromium from 15 to 30%, which makes it rust-resistant, creating a protective layer of oxide on the surface, and carbon. The most popular brands of this type are foreign. These are the 300th and 400th series. They are distinguished by their strength, resistance to adverse conditions and ductility. The 200 series is of lower quality, but cheaper. This is a beneficial factor for the manufacturer. Its composition was first noticed in 1913 by Harry Brearley, who conducted many different experiments on steel.
At the moment, stainless steel is divided into three groups:
- Heat-resistant - at high temperatures it has high mechanical strength and stability. The parts that are made from it are used in the pharmaceutical, rocketry, and textile industries.
- Rust-resistant - has great resistance to rusting processes. It is used in household and medical devices, as well as in mechanical engineering for the manufacture of parts.
- Heat-resistant - resistant to corrosion at high temperatures, suitable for use in chemical plants.
Melting cast iron
The material has excellent casting properties, has good fluidity, and its melting point is significantly lower when compared with steel and ductile iron. Such properties are taken into account when giving shape.
To connect the material to brass, in most cases, gaseous flux is used. Cast iron rods coated with copper can also be used, which has a good effect on the wettability of the edging with the deposited metal. For rods, eutectic cast iron is used, the melting point of which ranges from 1050-1200 degrees .
Welding can also take place using paste-like fluxes. When there are no special rods made of cast iron or brass L-62, then cracks in elements made of this material can be eliminated by using wire, the main component of which is electrolytic red copper.
Significantly above the melting point, the material overheats, which leads to the dissolution of suspended particles. They do not always dissolve completely, but graphite still forms with difficulty. In some cases, it occurs if additional substances are added to cast iron that affect the formation of additional graphite crystallization centers.
Cast iron has excellent casting qualities when compared to steel, which makes working with it convenient. Good fluidity and mold fillability are ensured by a lower melting point and the final crystallization process at constant temperatures.
These advantages suggest that cast iron is a valuable structural material, which is actively used in the production of machine parts (if there are no significant tensile and impact loads).
Semi-synthetic cast iron is melted by melting the charge; the temperature range is 1400−1450 degrees . After the melting of the charge is completed, the material is stored in the furnace crucible at slight overheating (the melting temperature should not be exceeded by more than one hundred degrees). What should be done to create a slag cover? When the charge gradually melts, it is necessary to apply cullet or hot quartz sand to the metal mirror.
Types of welding
Gas welding is carried out by melting particles of joined elements and rods made of filler metals with a flame.
This welding is used to join metal parts, non-metallic components and alloys with unequal melting points. Their thickness should not exceed 30 mm. The device does not require electricity.
Electric arc welding is also widely . The electric arc promotes the active interaction of the melted metal with the metal of the electrode and the formation of a durable seam. To avoid oxidation, a special protective layer is applied to the seam.
Using electric arc welding, cast iron parts, structural steels, copper, aluminum and other alloys are connected.
Melting temperature, properties and independent melting of cast iron
Cast iron is an alloy based on iron and carbon. It differs from steel in the latter content – 2% or more. Some brands contain up to 4% carbon. Most often, an alloy with a carbon content of 3-3.5% is used.
This is a casting material. For such a metal, such properties as its melting point, as well as its thermal properties - heat capacity, thermal conductivity, thermal diffusivity - come to the fore. How different chemical elements affect the quality of this metal and whether it is possible to melt it yourself - this will be discussed in the article.
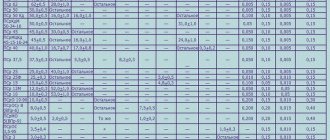
Characteristics table
Metals and alloys are an indispensable basis for forging , foundry, jewelry and many other areas of production. Whatever the craftsman does ( gold jewelry , cast iron fences, steel knives or copper bracelets) , in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum - 660 °C;
- copper melting point - 1083 °C;
- melting point of gold - 1063 °C;
- silver - 960 °C;
- tin - 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead - 327 °C;
- melting point of iron - 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) - from 1100 °C to 1300 °C;
- mercury - -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum - 2519 °C , for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
The maximum boiling point of metals is rhenium - 5596 ° C. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals . The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Another indicator found in the tables is the thermal conductivity of metals . Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
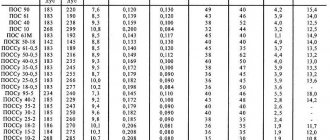
Thermal properties of cast iron
An important category of physical properties of a material is its thermal properties. These include:
- Heat capacity.
- Thermal conductivity.
- Thermal diffusivity.
- Thermal expansion coefficient.
They all depend on the composition, structure, and therefore on the grade of the alloy. In addition, these properties of the metal change with changes in its temperature (the so-called displacement rule). The nature of this dependence and the main physical properties are given in the table.
Heat capacity (s)
This is the amount of heat that must be supplied to the body in order for its temperature to increase by one Kelvin (hereinafter all values are converted to degrees Celsius).
The heat capacity depends on the composition of the alloy, as well as on temperature (T). The higher T, the greater the heat capacity. If the temperature is above T for phase transformations, but below T for melting, then
at T exceeding the melting point:
c = 0.23±0.03 cal/(G˚С)
Volumetric heat capacity (the ratio of heat capacity to the volume of a substance) for approximate calculations is accepted:
- cast iron in solid state c' = 1 cal/(cm3G˚C)
- molten c' = 1.5 cal/(cm3G˚C)
Thermal conductivity (λ)
This is a quantitative characteristic of a body's ability to conduct heat. The displacement rule does not apply to thermal conductivity. The temperature of the material increases – λ decreases. It depends on the composition of the alloy, and to a greater extent on its structure. Substances that increase the degree of graphitization increase thermal conductivity, and substances that prevent the formation of graphite decrease it.
Types of cast iron
There are several types of cast iron. Various alloying impurities are added to it, which change the characteristics of the solid material. For this, aluminum, chromium, vanadium or nickel are used. In addition to them there are other impurities. The parameters of finished products directly depend on the composition of the alloy. Varieties:
- Gray cast iron. It is considered the most popular type. The composition contains 2.5% carbon, which is a particle of graphite or perlite. Has a high strength index. Gray cast iron is used to make parts that can withstand constant loads. These can be gears, housing parts, bushings.
- White cast iron. The carbon contained in the composition is carbide particles. A white mark remains on the fracture of the material, which corresponds to the name. Carbon content averages more than 3%. A fragile and brittle type of material, which is why it is used only in static parts.
- Half-hearted. Combines the characteristics of the two previous types of cast iron. Graphite and carbide particles saturate the metal with carbon. Its content is from 3.5 to 4.2%. Wear-resistant material used in mechanical engineering. Withstands constant friction.
- Malleable cast iron. It is obtained from the second type of material, after annealing. The alloy contains carbon in the form of ferrite particles. Its amount is about 3.5%. Like half-shaft, it is used for the manufacture of parts in mechanical engineering.
To obtain a high-strength material, graphite particles are processed so that they take on a spherical shape and fill the crystal lattice. Magnesium, calcium or cerium are added to the alloy.
The influence of chemical elements on the properties of metal
What is it like?
The structure of cast iron is an iron base with graphite (carbon) inclusions. This material is distinguished not by its composition, but by the form of carbon in it:
- White cast iron (BC). Contains carbide (cementite) - this is a form of carbon, the same as in steel. It has a whitish color when scrapped. Very hard and brittle. In its pure form it is almost never used.
- Gray cast iron (GC). Contains carbon in the form of flake graphite. Such inclusions have a bad effect on the quality of the material. To change the shape of graphite grains, there are special methods of melting and further processing. Graphite in midrange can also be in the form of fibers (“worm-shaped” form) - the so-called vermicular graphite (from the Latin word vermiculus - a worm, like vermicelli).
- Highly durable. Spherical shape of graphite grains. It is obtained by introducing magnesium into the alloy.
- Malleable cast iron. To obtain it, warheads are annealed. Graphite grains in the form of flakes.
As a result, the main difference between it (apart from white) and steel is the presence of a graphite structure. And different forms of graphite determine the properties of different grades.
Conventionally, graphite grains are voids and cracks, and cast iron is steel riddled with microscopic cracks.
Accordingly, the more voids, the worse the quality of the metal. The shape and relative position of the inclusions also matters.
However, graphite grains should not be accepted as exclusively harmful. Due to the presence of graphite, this material is easier to machine and the chips become more brittle. In addition, it resists friction well also due to the graphite.
Impurities
Of course, this metal contains more than just iron and carbon. It contains the same elements as steel alloys - phosphorus, manganese, sulfur, silicon and others. These additives indirectly affect the characteristics of the alloy - they change the course of graphitization. The quality of the material depends on this parameter.
- Phosphorus. Has little effect on the formation of graphite. But it is still needed, because it improves fluidity. Solid inclusions of phosphorus provide high hardness and wear resistance of the metal.
- Manganese. It interferes with graphitization and, as it were, “bleaches” cast iron.
- Sulfur. Like silicon, it promotes bleaching of the metal, and also impairs fluidity. The amount of sulfur in the alloy is limited. For small castings no more than 0.08%, for parts more - up to 0.1-0.12%.
- Silicon. Strongly affects the properties of the material, increasing graphitization. The metal can contain from 0.3-0.5 to 3-5% silicon. By varying the amount of silicon, an alloy with different properties is obtained - from white to high-strength.
- Magnesium. Helps to obtain material with spherical grain shape. The boiling point of magnesium is low (1107˚C). For this and other reasons, introducing magnesium into the alloy is difficult. To avoid its boiling, the material is smelted using various methods of introducing magnesium.
In addition to the usual impurities, cast iron may contain other substances. This is the so-called alloyed material. Chromium, molybdenum, vanadium interfere with the process of graphite formation. Copper, nickel and most other substances contribute to graphitization.
Self-smelting technology
Non-industrial smelting of cast iron is a very labor-intensive process. It is impossible to smelt factory-quality castings with your own hands in artisanal conditions.
You cannot smelt this metal at home. You need a separate ventilated room - a garage, for example. Melting is carried out in furnaces. In industry, blast furnaces, cupola furnaces and induction furnaces are used.
A blast furnace is an industrial unit capable of melting metal on a huge scale. Iron ore raw materials can be smelted in it. After launch, it works without interruption for up to 5-6, or even up to 10 years. Then it is stopped, serviced and started again. Melting of the metal takes place in the presence of gases to improve the quality of the material. Such ovens are not suitable for small and medium-sized production. Fuel - coke.
A cupola furnace is a shaft-type furnace, like a blast furnace. It differs from the latter in that it does not maintain a special composition of gases. It is not ore that is smelted in it, but scrap iron. It is more suitable for small production.
An induction furnace is a modern type of equipment. The smelting process in such a furnace can be controlled, temperature, heating time and charge composition can be adjusted.
Melting is carried out in crucibles made of refractory clay or brick. Steel ones are not suitable, although steel begins to melt at a temperature higher than cast iron. Flux is required - a substance that promotes the formation of low-melting slag. For example, limestone (CaCO3), fluorspar (CaF2). To obtain gray rather than white cast iron, ferrosilicon (an alloy of iron and silicon) is added to the charge. It improves the formation of graphite grains. Once melted, the metal is poured into a sand or metal mold.
Metal casting is an explosive and fire hazardous job. In addition, it is necessary to have certain knowledge in the field of metallurgy. To organize production, you will need to complete documentation, pass inspections, obtain permission and a license to work.
Production technology
Cast iron has been smelted for several decades, which is due to its unique performance qualities. The large number of varieties of alloys determines the application of special marking rules. Marking of cast iron is carried out as follows:
- Foundries are designated by the letter L.
- Gray has become widespread; the combination of letters “SCH” is used to designate it.
- Malleable is designated KCH.
- Extreme or white is designated by the letter P.
- Anti-friction or gray indicate ASF.
- Alloy cast irons can have a wide variety of chemical compositions and are designated by the letter “C”.
The technology of cast iron production involves several stages that make it possible to obtain the required structure. Considering the process of producing cast iron, we note the following points:
- Production is carried out in special blast furnaces.
- Alloyed and heat-resistant cast iron can be obtained by using iron ore as a raw material.
- The technology is presented in the reduction of iron oxides ore. As a result of the restructuring of the crystal lattice and changes in the structure, the output is a material called cast iron.
- Considering production methods, we note that the features of the technology also lie in the materials used - cokes. Coke refers to natural gas or thermoanthracite, which acts as fuel.
- The production of cast iron involves tempering iron in solid form using a special furnace. At this stage, liquid cast iron is obtained.
Equipment for the production of cast iron can vary significantly. In addition, the production technology used largely determines what kind of material will be obtained. An example is the production of ductile iron, which involves giving the structure an unusual shape.
Melting temperature of cast iron, steel melting
In industry and everyday life, cast iron products are widely used. The metal is iron, which has 2 percent carbon integrated into its molecular structure. Today, many grades of metal are produced that have different fracture characteristics. About a hundred species.
Production requires a huge amount of thermal energy, since the melting point of cast iron is over one thousand degrees Celsius. Melting occurs at a temperature of 1150 - 1200 C°.
In addition to carbon, to obtain the required grade, silicon, sulfur, manganese, and phosphorus are added to the batches. Increased strength is achieved by incorporating alloying additives into the batches.
Differences from steel
According to the technological process, cast iron is the primary product obtained by casting, and steel is the final product. The molecular structure of steel contains carbon in an insignificant amount. The material is plastic and lends itself well to mechanical processing. Products are manufactured by forging, welding, and rolling in mills. Has a high melting point. According to technology, steel is subject to hardening. The quality depends on the prepared mixture and on the melting temperature of the steels.
The rate of transformation of steel into a liquid state depends on various additives. A specific answer to the question at what temperature steel melts can be conditionally given only the heating range. The transition from a solid to a liquid consistency occurs at a temperature of 1450-1600 C°. The given digital parameter indicates the difference between steel and cast iron. These are different melting temperatures.
Cast iron is not as strong as steel. Cast blanks contain pores, making them brittle. It is during the casting process that cast iron products are produced. The presence of microscopic voids reduces the thermal conductivity characteristics of the metal. It is important to set the thermal regime, to record at what temperature cast iron melts.
Ferrous metallurgy produces several types of primary products. Let's look at some of them.
Grayish cast iron
Alloys formed by iron and carbon components change structure when integrating flake, flake, and fibrous graphite. Manufacturers obtain high-strength cast iron by adding globular graphite. The presence of Mg, Ce (magnesium, cerium) in the batch motivates its modification. Depending on how quickly molten cast iron cools, it acquires new consumer characteristics. Products of the required quality are obtained from a skillful combination of specific properties.
To make it easier to find the right material in catalogs, products are marked with the abbreviation S. Ch. The numbers following the letters indicate the force load limit in kilograms/per square millimeter. High-strength metal has the letter designation V.Ch. The numbers indicate the amount of strength, and also separated by a hyphen - the increase in length as a percentage. For example, HF60−1
Gray cast iron has excellent technological characteristics during its production process:
- Crystallization does not require extreme temperatures, which has a positive effect on saving electricity and other types of energy.
- Shows unique liquid fluidity.
- When poured, it exhibits optimal shrinkage.
Due to its unique properties, metal is the base material for the production of products.
Has disadvantages in application. They manufacture units and parts that work only in compression. Machine beds, cylinders, various pistons, and so on are cast. Critical fragility indicators do not allow use for the production of products operating under conditions of bending forces. Melting point 1150 - 1260 C°
Bleached fabric colors
White cast iron contains an iron-carbon compound called cementite. It has colossal hardness, excluding plasticity. If you break a metal, the color is visible on the break. Cast iron is harder than stone and as fragile as eggshells. Subjected to processing to obtain a malleable variety. The melting point occurs in the range of 1150 – 1350 C °. It is appropriate to note that the term malleable is used conditionally, since the metal cannot be processed plastically. Malleable cast iron is produced by thermal firing.
Heating the material above 900 degrees Celsius affects its properties. The rapid cooling of graphite also leads to this result. Failure to comply with technological parameters leads to complications in the production of welding work and processing of workpieces.
High strength cast iron
In ferrous metallurgy, high-strength material is called cast iron, which has graphite inclusions in its molecular structure, the shape of which is spheroidal. The unique ratio of spherical graphite surface to volume ensures the formation of a metal base, that is, it affects strength. Melting the metal with the integration of spheroidal graphite does not allow cracks. New properties of the metal are formed: it becomes strong when subjected to bending force. In addition, it demonstrates:
- instantaneous impact viscosity;
- increase in turnover rate;
- slight elongation, which can be called a relative phenomenon.
- unique resistance to compression;
- wear resistance.
This type can be welded. The metal connection is carried out using fluxes used in the form of paste-like consistencies.
Heavy duty cast iron material has excellent casting properties. Excellent fluidity in the liquid state ensures exemplary filling of molds. According to some technological parameters, the material can be compared with steel.
Taking into account the excellent structural properties, factories produce parts for components and systems if they do not experience tensile loads during operation of machines and mechanisms.
Cast iron - composition, properties and characteristics
The term “cast iron” can mean both a structural material based on iron and a metal vessel, a round pot for cooking. The latter is rare. Modern dishes are crowding.
The word “cast iron” is completely outdated. This is what the railway was called in the 19th and early 20th centuries.
What is cast iron
It is an alloy of iron and carbon
with the content of the latter from 2.14%. Ideally. In fact, in addition to those indicated, there are always impurities and alloying elements. So the distinction “floats”.
Depending on the carbon content relative to the eutectic, metal varieties are distinguished. Eutectic is the composition of an alloy with a minimum melting point.
For cast iron, the carbon content is approximately 4.3%. Why “approximately” has already been said. Therefore, it is customary to divide cast iron into:
- hypoeutectic - 2.14 - 4.3% carbon;
- eutectic - 4.3% carbon;
- hypereutectic - from 4.3 to 6.67% carbon.
Types of cast iron
In the generally accepted classification, they are divided according to the form of carbon they contain.
White
It is called so because of the characteristic color of the chip. Carbon C is contained in the form of cementite
(formula Fe3C), formed during cooling of the melt. Hard refractory material.
In hypoeutectic alloys - in the composition of pearlite and ledeburite. In eutectic ones - in ledeburite. In hypereutectic - primary cementite and ledeburite.
In its original form, such cast iron is practically not used. Cannot be processed with high-speed steel tools. Only with carbide nozzles (VC), and even then with difficulty.
It is used as a raw material for producing malleable.
Grey
Also named after the shade on the chip. Contains graphite fractions of various shapes. Carbon precipitation is facilitated by the addition of silicon.
Properties and structure strongly depend on cooling conditions after crystallization.
Rapid cooling will give a predominance of perlite. Alloy of ferrite and carbide. A kind of “hardening” will increase strength and hardness. And fragility, which is not always acceptable.
Gentle cooling determines the increase in ferrite content. An alloy of iron with oxides, mainly Fe2O3. Plasticity will improve. Therefore, modes are selected based on the required parameters.
Gray cast iron is suitable for cast structures.
It has a low curing temperature and good fluidity. Not prone to shell formation.
With all this, carbon inclusions cause low crack resistance. The material confidently withstands compressive forces, but is completely unsuitable for stretching/bending.
The marking indicates the symbols SCh and the ultimate strength in kg/mm2: SCh25. The most common cast irons are those with a C content below 3.7%.
Malleable
To produce white cast iron, it is heated to the required temperature, kept for a sufficient time and cooled slowly (“annealing”). The process provokes the decomposition of Fe3C with the release of graphite and the appearance of ferrite.
The shape of carbon inclusions is not similar to those in gray cast iron. This explains the appearance of some tensile strength and toughness.
Marked “KCh” with the addition of permissible tensile strength in MPa x 10-1 and maximum elongation. Example: CC 35-11.
High strength
A type of gray cast iron, only the graphite formations are shaped like balls. The roundness of the inclusions makes the crystal lattice not prone to cracks.
As a result, the initially valuable properties of cast iron (resistance to compression, ease of casting, etc.) are supplemented by a tensile yield strength comparable to steel, and crack resistance and ductility appear.
They are marked similarly to malleable ones, but with the designation “HF”.
Peredelny
Used as a raw material for steel smelting. Often it does not even leave the enterprise where it was made.
Special
The production of such brands is small, up to 2% of the total volume. May contain significant amounts of alloying elements. Designed for limited purposes and specific conditions. Corrosion and chemically resistant ferroalloys are common.
One of the varieties is anti-friction cast iron. Used for the manufacture of rubbing parts. Alloyed primarily with chromium. Nickel, titanium, copper and others are also added.
It is characterized by high hardness (up to HB 300) and low coefficient of friction (up to 0.8 in the absence of lubricating emulsions).
Base materials: gray, ductile and high-strength cast iron. The markings are respectively AChS, AChK, AChV. The digital components are described above.
Advantages and disadvantages of the material
It is worth discussing in comparison with steel, although low-quality carbon steel is essentially the same as cast iron.
In some parameters (density, magnetic properties, typical chemical reactions) ferroalloys are almost identical. There are significant differences in the technology of use.
Advantages:
- Moderate cost.
Carbonation is part of the smelting process from ore. A decrease in its content will inevitably increase the price of the metal.
- Excellent casting qualities.
The melt is fluid. With low crystallization shrinkage, which minimizes defects. Relatively low melting point.
- The products are durable, have a hard surface, and are wear-resistant.
- The compositions used in mechanical engineering can be processed by cutting.
- Durable.
Including plumbing and sewer parts.
- Items that have become unnecessary are easy to recycle. Any reception point with hands will be torn off.
Flaws:
- Due to the high carbon content it is fragile. Not very suitable for pressure treatment. Certain brands produce forged products of excellent quality. But this is rather piece work and unprofitable on an industrial scale.
- Welding is allowed only in extreme cases. The technology is quite complex, and the risk of defects is high.
- The products are always massive. A thin-walled structure will not work, since it will not support its own weight and will not be able to be manufactured.
- Easily oxidizes in a humid environment. It will not rust through due to its inevitable monumentality, but it will take on an unkempt appearance. Parts located outdoors require a corrosion-resistant coating.
Iron production
People mastered the rudiments of ferrous metallurgy already in the 2nd millennium BC. e. To obtain steel. But blast furnaces appeared in Europe only in the 14th and 15th centuries. Pig iron was obtained as a waste by-product.
They appreciated it when they noticed its outstanding casting qualities. It is convenient for making cannonballs, and it is also more convenient to obtain steel from it.
Technology reached Russia in a meaningful way in the 17th century. This happened under Peter I, when they were looking for material for weapons.
Iron ore is usually used as a raw material. The highest yield is obtained from magnetic and red, abundantly containing Fe.
Coke is used to maintain temperature. Combustion air is supplied forcibly. Flux (limestone) is designed to supply carbon dioxide. Main reaction:
.
The reduced Fe is lowered into the furnace, where it is saturated with carbon. The furnace operating cycle is continuous.
Getting steel
About 85% of cast iron is used for further steel production. An open hearth furnace is used for smelting.
During the melting process of the loaded raw material, a significant mass of FeO oxide is formed. As it heats up, the following reaction occurs:
.
Excess carbon is removed.
Electric arc and induction furnaces are also used.
Areas of use
Due to the modern tendency to make equipment as light as possible, cast iron is used less and less.
But there are areas where it is still indispensable and cost-effective:
- In mechanical engineering it is used for large body parts with low tensile loads. Beds for machine tools, cylinder blocks for internal combustion engines. Flywheels, pulleys, gears, hydraulic cylinders, gear housings, electric motors, pistons.
- Plumbing fittings, sewer pipes.
- Decorative elements: fences, grilles, gates.
- Stoves for houses, baths.
Grid changes
With increasing heat (cast iron melts at a temperature of 1200 degrees Celsius), the crystal lattice transitions into the current liquid state. It is at this moment that the internal energy of the metal increases. Having reached heating above one thousand degrees, the crystal lattice is destroyed. At this time, incoming thermal energy continues to weaken molecular bonds. There is an increase in energy reserves inside the metal. It is several times higher than that containing crystallized material.
The cessation of heating is the beginning of cooling of the metal. A reverse crystallization process occurs, developing according to a dendritic algorithm. That is, from the points that motivate such development. They (dendrites) act as a priori stages of the process. The crystal grows, as it were, from the center of the phenomenon. In liquid, but already cooling cast iron, crystallization occurs according to the principle of the structure of wood. The process involves dendrites of cementite, austenite and graphite. It has been established thermodynamically that it is spherical graphite that is represented by a dendrite having a sectoral layered structure.