08/14/2020 Author: VT-METALL
Issues discussed in the material:
- Useful and special impurities in steel
- Harmful impurities in steel that impair its properties
Harmful impurities in steel not only worsen its composition, but can also lead to subsequent deformation of a product made from it. However, they cannot all be considered undesirable. Some of them are considered useful, while others cannot be gotten rid of at all, since they are permanent. And there is no need to eliminate them, since permanent impurities can affect the quality characteristics of steel.
In this article we will talk about what harmful impurities are in steel and how they affect its composition and the characteristics of steel products.
Impurities: permanent, hidden and random
Manganese, silicon, aluminum, sulfur and phosphorus
are classified as
permanent impurities
.
Aluminum, together with manganese and silicon, is used as a deoxidizer and therefore they are always present in small quantities in deoxidized steels. Iron ores, as well as fuels and fluxes, always contain a certain amount of phosphorus and sulfur, which remain in cast iron and then pass into steel
.
Nitrogen
called
a hidden
impurity - it enters steel mainly from the air.
To random
impurities include
copper, arsenic, tin, zinc, antimony, lead
and other elements. They end up in the steel with the charge - with ores from various deposits, as well as from iron scrap.
All impurities - permanent, hidden and accidental - are inevitable to varying degrees due to steel production technology. Thus, mild steel usually contains these impurities within the following limits: 0.3-0.7% manganese; 0.2-0.4% silicon; 0.01-0.02% aluminum; 0.01-0.05% phosphorus, 0.01-0.04% sulfur, 0.-0.2% copper. In these quantities, these elements are considered as impurities, and in larger quantities, which are intentionally added to steel, they are already considered alloying elements.
Useful and special impurities in steel
Steel contains harmful and beneficial impurities. First, let's look at the useful ones, which include manganese and silicon:
- Manganese is a chemical element that increases the hardenability of steel and reduces the influence of sulfur, which has a harmful effect on the metal.
- Silicon - an admixture of this element helps to deoxidize steel and, as a result, increase its strength. It is specially added to the metal during its smelting.
Carbon steel contains an admixture of silicon of no more than 0.35–0.4% and manganese in an amount of 0.5–0.8%. The transition of manganese and silicon into steel occurs during deoxidation during smelting. These chemical elements combine with the oxygen of iron oxide FeO, and then, turning into oxides, pass into slag, that is, in other words, they deoxidize the steel.
This process has a beneficial effect on the properties of steel. Due to degassing of the metal by silicon, its density increases. Part of the chemical element remains in the ferrite (solid solution) after deoxidation, which leads to a significant increase in the yield strength. At the same time, the ability of steel to cold heading and drawing is reduced.
Recommended reading
- Cutting copper with a laser: advantages and disadvantages of technology
- Types of metal cutting: industrial applications
- Metalworking according to drawings: convenient and profitable
For this reason, manufacturers are reducing the amount of silicon in steels produced for cold forming and heading. The strength of the metal is significantly increased due to the admixture of manganese. The latter greatly reduces the red brittleness of steel, leaving ductility almost unchanged. Thus, the brittleness of steel sharply decreases when exposed to high temperatures, which arose due to the presence of sulfur.
To obtain steels with certain properties, special impurities are added to the metal. They are called alloying elements. Steels are called alloyed steels.
Let us dwell in detail on the purpose of some elements:
- Aluminum - its admixture helps to increase the scale and heat resistance of steel.
- Copper – increases the resistance of steel to corrosion.
- Chromium – increases the strength and hardness of steels, increases resistance to corrosion, while ductility decreases slightly. What makes stainless steel stainless is its high chromium content.
- Nickel – increases ductility, strength, and makes steel resistant to corrosion.
- Tungsten – when added to steel, creates corbides (chemical compounds of increased hardness). They significantly increase red resistance and hardness. Under the influence of tungsten, steel stops expanding during heating, and its brittleness disappears when tempered.
- Vanadium – contributes to an increase in the density, strength and hardness of steel. It is recognized as an excellent deoxidizing agent.
- Cobalt - under its influence, heat resistance, resistance to shock loads increases, and magnetic properties increase.
- Molybdenum – steel’s resistance to oxidation improves when exposed to high temperatures, elasticity and red-hardness increases, corrosion resistance increases, and tensile strength increases.
- Titanium - being an excellent deoxidizer, it increases resistance to corrosion, increases the density and strength of the metal, and improves its workability.
- Cerium – helps to increase the ductility and strength of steel.
- Zirconium (Z) - affects the grain size of steel, making it possible to produce metal with a specified grain size, making it finer.
- Lanthanum, neodymium and cesium - reduce the porosity of steel, reduce the amount of sulfur, make the surface quality better and the grain finer.
The influence of phosphorus on the properties of steels
Phosphorus (P) segregates during steel solidification, but to a lesser extent than carbon and sulfur. Phosphorus dissolves in ferrite and thereby increases the strength of steels. As the phosphorus content in steels increases, their ductility and toughness decrease and their tendency to cold brittleness increases.
The solubility of phosphorus at high temperatures reaches 1.2%. With decreasing temperature, the solubility of phosphorus in iron drops sharply to 0.02-0.03%. This amount of phosphorus is typical for steels, that is, all phosphorus is usually dissolved in alpha iron.
Phosphorus has a strong tendency to segregate at grain boundaries, resulting in temper brittleness in alloy steels, especially in manganese, chromium, magnesium-silicon, chromium-nickel and chromium-manganese steels. Phosphorus, in addition, increases the hardening of steels and, like silicon, slows down the decomposition of martensite in steels.
Increased phosphorus content is often specified in low-alloy steels to improve their machining, especially automatic machining.
In low-alloy structural steels with a carbon content of about 0.1%, phosphorus increases strength and resistance to atmospheric corrosion.
In austenitic chromium-nickel steels, phosphorus additions help increase the yield strength. In strong oxidizers, the presence of phosphorus in austenitic stainless steels can lead to grain boundary corrosion. This is due to the phenomenon of phosphorus segregation along grain boundaries.
Useful impurities in steel
If we talk about substances that improve the characteristics of the metal, these include:
- chromium – increases corrosion resistance and hardness; with a high chromium content, steel becomes stainless;
- nickel – increases the strength and ductility of the alloy;
- aluminum – increases heat resistance and resistance to scale formation;
- tungsten – increases the red resistance and hardness of the metal due to the ability to form carbides (superhard compounds);
- cobalt – increases heat resistance and impact resistance;
- titanium – reduces the content of harmful oxygen, increases corrosion resistance and machinability;
- vanadium - like titanium, is considered an effective deoxidizer, increases the density and strength of the metal.
The list is by no means exhaustive; there are other elements that improve the quality of steel. They are used in the smelting of alloy, stainless and heat-resistant steels. You can learn about the composition of the metal from the designation of a particular grade of steel.
The influence of sulfur on the properties of steels
The sulfur (S) content in high-quality steels does not exceed 0.02-0.03%. In general purpose steels, the permissible sulfur content is higher - 0.03-0.04%. By special treatment of liquid steel, the sulfur content in steel is brought to 0.005%.
Sulfur does not dissolve in iron, so any amount of it forms iron sulfide FeS with iron. This sulfide is part of the eutectic, which is formed at 988 °C.
An increased sulfur content in steels leads to their red brittleness due to low-melting sulfide eutectics that arise along grain boundaries. The phenomenon is red
brittleness occurs at a temperature of 800 °C, that is, at the
red-hot
of steel.
Sulfur has a detrimental effect on the ductility, toughness, weldability and surface quality of steels (especially in steels with low carbon and manganese content).
Sulfur has a very strong tendency to segregate at grain boundaries. This leads to a decrease in the ductility of steels in the hot state. However, 0.08 to 0.33% sulfur is deliberately added to steels for automatic machining. It is known that the presence of sulfur increases the fatigue strength of bearing steels.
The presence of manganese in steel reduces the harmful effects of sulfur. In liquid steel, the formation reaction of manganese sulfide occurs. This sulfide melts at 1620 °C - at temperatures much higher than the temperature of hot working of steels. Manganese sulfides are plastic at temperatures of hot processing of steels (800-1200°C) and therefore are easily deformed.
Classification of steel types
Definition 1
Steel is an alloy of iron with carbon and other chemical elements, which contains a minimum of 45% iron and a carbon content ranging from 0.02% to 2.14%.
If the carbon content in steel is from 0.6% to 2.14%, then it is high-carbon. If the carbon content is more than 2.14%, then the alloy is called cast iron. Modern powder steels can contain up to 3% carbon, but they are not cast iron. Carbon reduces toughness and ductility and gives the alloy hardness and strength.
Steels with high elastic properties are widely used in instrument and mechanical engineering. In mechanical engineering they are used to produce power springs for various purposes, shock absorbers and leaf springs. In instrument making, steels are used in the manufacture of relay plates, membranes, springs, suspensions and braces. Steels can be classified according to the following criteria:
Are you an expert in this subject area? We invite you to become the author of the Directory Working Conditions
- Structure. According to this characteristic, steels are divided into pearlitic, austenitic, bainitic, martensitic and ferritic.
- Purpose. According to this criterion, steels are divided into stainless steel, tool steel, structural steel, cryogenic steel and heat-resistant steel.
- Chemical composition. According to this characteristic, steels are divided into alloy and carbon. Carbon ones are divided into low-, medium- and high-carbon, alloyed into low-, medium- and high-alloy.
- Method of receipt. According to this characteristic, steels are divided into steels of ordinary quality, high-quality, high-quality and especially high-quality.
The main characteristics of steel include: density (from 7700 to 7900 kilograms per cubic meter), specific gravity, specific heat capacity, melting point, specific heat of fusion, thermal conductivity, coefficient of linear thermal expansion and tensile strength.
The influence of aluminum on the properties of steels
Aluminum (Al) is widely used for deoxidation of liquid steel, as well as for grain refinement of steel ingots. The harmful effects of aluminum include the fact that it promotes the graphitization of steels. Although aluminum is often considered an impurity, it is also actively used as an alloying element. Because aluminum forms solid nitrides with nitrogen, it is usually an alloying element in nitrided steels. Aluminum increases the resistance of steels to scaling, and therefore it is added to heat-resistant steels and alloys. In dispersion-hardening stainless steels, aluminum is used as an alloying element that accelerates the dispersion precipitation reaction. Aluminum increases the corrosion resistance of low-carbon steels. Of all the alloying elements, aluminum is the most effective for controlling grain growth when heating steels for hardening.
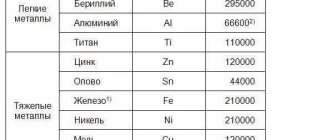
Scrap metal
According to the classification of N. T. Gudtsov, impurities in steel are divided into permanent (ordinary), and random, uncovered (harmful).
Constant impurities in steel are manganese and silicon, which are present as impurities in almost all industrial steels. The manganese content in structural steels is usually in the range of 0.3-0.8% (if manganese is not an alloying element), in tool steels its content is slightly less (q 15 0.40%). The introduction of manganese as a technological additive in such quantities is necessary to convert sulfur from iron sulfide to manganese sulfide. Silicon in well-oxidized (quiet) steels is usually contained in the range of 0.17-0.37%. In incompletely deoxidized low-carbon (^0.2% C) steels it is contained less: in semi-quiet steels 0.05-0.017%, in boiling steels <0.07%. In stainless and heat-resistant steels not alloyed with silicon, it can contain up to 0.8%.
Random impurities in steel can be almost any element that accidentally gets into the steel from scrap, natural alloy ore or deoxidizers. Most often these are Cr, Ni, Cu, Mo, W, Al, Ti, etc. in quantities limited for impurities.
Hidden impurities in steel are sulfur, phosphorus, arsenic and the gases hydrogen, nitrogen and oxygen. However, recently, nitrogen, sulfur, and phosphorus are sometimes used as alloying additives to provide a number of special properties of steels. N
Based on the grade chemical composition of the steel, it is possible to determine which elements are alloying additives and which are impurities. If the brand chemical composition of steel sets the lower (no less) and upper (no more) limits for the content of a given element in the steel, then it will be an alloying element. As a rule, only the upper content limit is set for impurities. The only exceptions are Manganese and silicon, the amount of which is regulated by lower and upper limits for both impurities and alloying additives.
Harmful impurities: sulfur, phosphorus and gases are present in almost all steels and, depending on the type of steel, they can have different effects on the properties. Let's consider their role in steel.
Sulfur
At room temperature, the solubility of sulfur in a-jelly-ze is practically absent. Therefore, all the sulfur in steel is bound into iron and manganese sulfides and partially into sulfides of alloying elements. With increasing temperature, sulfur dissolves in a- and y-iron, although only slightly, but up to quite certain concentrations (0.02% in a-iron at 913°C and 0.05% S in v-iron at 1365°C) . Therefore, sulfur inclusions can change during heat treatment of steel.
If sulfur is bound into iron sulfide FeS at relatively low temperatures of hot deformation of steel due to the melting of the iron sulfide eutectic (988°C), red brittleness of the steel is observed. At higher temperatures of hot plastic deformation, hot brittleness of steel is possible, caused by the melting of austenite located along the boundaries of the primary grains, iron sulfide itself (1188 ° C). The introduction of manganese into steel in the ratio Mn:S>8-10 leads to almost complete binding of sulfur in a tight
Rice. 9. Dependence of the impact strength of normalized steel type 45 on the sulfur content in it (W. Knorr)
O, iz o,/J 0.01 o, og O1OJ
S, (by mass]
Rns. 10. Dependence of impact strength KCV (a) and transition temperature Tu, (b) of 08G2MB steel on sulfur content (E. N. Zhukova) oMP a:
Effect or sulfide paradox (Fig. 10). It is explained by the fact that an increase in sulfur content reduces the impact toughness of transverse samples with a sharp notch (KCV), i.e., the steel’s resistance to ductile fracture (Fig. 10a). Increasing the strength of steel leads to a more significant effect of sulfur on reducing toughness. The resistance to ductile fracture decreases most intensively at sulfur contents of up to 0.010%. At the same time, the influence of sulfur on the temperature of transition from a ductile to a brittle state, determined by the presence of 50% of the ductile component in the fracture of impact samples—T5о, i.e., on the resistance of steel to brittle fracture, is extreme. As the data presented in Fig. 10.6, steel is most prone to brittle fracture at a sulfur concentration of ~0.010%. At lower and higher sulfur concentrations, the transition temperature T50 decreases. The extreme sulfur content of different steels may vary. Thus, the sulfide effect is to increase the steel's resistance to brittle fracture while reducing its resistance to ductile fracture as the sulfur content increases above a certain limit. It can be assumed that the sulfide effect is due to different interactions of a moving crack with sulfides depending on the viscosity of the matrix.
In heat-resistant austenitic steels, an increase in sulfur content noticeably reduces the limits of creep and long-term strength, i.e. S reduces the heat-resistant properties.
Phosphorus
The solubility of phosphorus in a- and y-iron is significantly higher than the phosphorus content in steel as an impurity. Therefore, phosphorus in steel is entirely in solid solution, and its effect on properties is reflected by changing the properties of ferrite and austenite. The harmful effect of phosphorus on properties can be aggravated due to its strong tendency to segregation (the degree of segregation reaches 2-3).
The effect of phosphorus on the properties of ferrite is manifested in its strengthening effect and especially in increasing the cold brittleness of steel, i.e., increasing the temperature of transition from a ductile to a brittle state (Fig. 11). \
Phosphorus is a strong hardening agent (see Chapter IV, paragraph – 4). Despite the fact that its content in steel is usually
Does not exceed 0.030-0.040%, it increases the yield strength of ferrite by 20-30 MPa. At the same time, an increase in phosphorus content within hundredths of a percent can cause an increase in the cold brittleness threshold by a non-
•m
?,% (by mass)
Rice. 11. The effect of phosphorus on Oa and at (M. S. Mikhalev, M. I. Goldshteii) and impact strength KCU (A. P. Gulyaev) of low-carbon ferrite-pearl steel (0.2% C, 1% Mn)
How many tens of degrees (~ 20-25 cC per 0.01% P) due to a strong reduction in the work of crack propagation.
In structural temperable steels, phosphorus is responsible for the manifestation of reversible temper embrittlement (see.
Ch. IX, paragraph 6). In this case, its influence on the cold brittleness threshold is especially strong (0.010% P increases the transition temperature by ~40°C).
Similarly, phosphorus affects the cold brittleness threshold of aus-taenitic manganese steels, while its harmful effect is less pronounced and less pronounced (Fig. 12). The influence of phosphorus, within acceptable limits, on the mechanical and heat-resistant properties of chromium-nickel austenitic stainless and heat-resistant steels is not noticeably manifested.
Rns. 12. The influence of phosphorus on the cold-brittleness threshold Tso of austic manganese steel g 110G13 (A.P. Gulyaev):
1 - cast; 2 - forged
Steels smelted from Kerch ores contain arsenic. Its effect on the properties of steel is similar to phosphorus, but the harmful effect of arsenic is much weaker than that of phosphorus. Therefore, in high-quality steel of such production, up to 0.08% As is allowed.
Gases in steel
In steels, hydrogen, oxygen, and nitrogen are usually present in certain quantities. Their content in steel depends primarily on the smelting method. Approximate content, %, of gases in steel for different smelting methods according to A.P. Gulyaev:
Electric Furnace Open Hearth Oxygen-
Main converter
Hydrogen • • • Oxygen • • • Nitrogen. . . •
. 0,0004—0,0006 0,002—0,004 0,007—0,010
0,0003—0,0007 0,005—0,008 0,004—0,006 0,0001—0,0008 0,005—0,003 0,002—0,005
Hydrogen can be part of a solid solution of steel and be released in a gaseous state, accumulating in the pores of the metal, and flakes are formed in the steel. Oxygen is usually bound into non-metallic inclusions. Nitrogen negatively affects the properties of steel if it is in solid solution or forms iron nitrides, causing aging of the steel. The positive effect of nitrogen on the properties of steel is manifested when it is bound into strong nitrides AlN, VN1 NbN or carbonitrides V (C, N), Nb (C, N), etc., which is used in steels with carbon nitride strengthening. In addition, nitrogen is widely used as an austenitizing element in corrosion-resistant and heat-resistant steels.
In conclusion, it should be noted that the fight against harmful impurities in steel is mainly carried out during steel smelting. Reducing the content of harmful impurities in steel often requires considerable costs for the implementation of certain technological methods and the use of special smelting methods.
The influence of nitrogen on the properties of steels
The harmful effect of nitrogen (N) is that the rather large, brittle non-metallic inclusions it forms - nitrides - worsen the properties of steel. A positive property of nitrogen is that it is capable of expanding the austenitic region of the phase diagram of steels. Nitrogen stabilizes the austenitic structure and partially replaces nickel in austenitic steels. Nitride-forming elements vanadium, niobium and titanium are added to low-alloy steels. When controlled by hot working and cooling, they form fine nitrides and carbonitrides, which significantly increase the strength of the steel.
The influence of copper on the properties of steels
Copper (Cu) has a moderate tendency to segregate. The harmful effects of copper include a decrease in the cold brittleness of steel. With an increased copper content, it negatively affects the quality of the steel surface during hot processing. However, with a content of more than 0.20% copper increases its resistance to atmospheric corrosion, as well as the strength properties of alloy and low-alloy steels. Copper in amounts greater than 1% increases the resistance of austenitic stainless steels to sulfuric and hydrochloric acids, as well as their resistance to stress corrosion.