History of discovery
The incredible qualities of the alloy were discovered in 1903 by an engineer from Germany who worked in the city of Duren. The name “duralumin” comes from this name. Duralumins are alloys that are distinguished by high strength and low weight, as well as other sought-after qualities.
Interesting: In 1911, at the St. Petersburg exhibition, such an alloy was awarded a silver medal in the category of the best materials used in aircraft.
Duralumin brought great benefits during the Great Patriotic War. It was used to make components for weapons, armored vehicles and military aircraft.
Over time, the composition of the material was updated, and new types of alloy were born.
Chemical composition
The composition of duralumin mainly consists of two metals.
The main part of duralumin is aluminum. Its share can be up to 94% of the total weight. The second most important element that is usually available is copper. The mass of other components is small. Additionally, the duralumin formula may contain magnesium, manganese, iron, and other metals. Composition of the popular alloy grade D16T:
- aluminum - 93-94%;
- copper - 3.8-4.9%;
- alloying alloys - 1.5-2%.
Industrial production
For industrial production of the alloy, high-power electricity is used.
To obtain duralumin, a compound (charge) is made - these are particles of different metals; later they will be fused into a homogeneous material. After this, the component is heated to a level of +500 ° C, then sharply cooled with water or nitrate. When the temperature of the duralumin workpiece reaches room temperature, hardening is done.
Interesting: Types of metal cutting equipment
Following this, the so-called “artificial aging” of the manufactured component is most often used. To do this, the material is additionally kept at high temperature for a long period: about 2 hours at +…+200° C. The process is carried out taking into account the brand of the mixture and the required properties. The aging procedure is carried out in order to obtain high strength duralumin. If this process is not applied, the metal will be soft and pliable.
Once formed, the component is sometimes coated with a protective substance that protects against corrosion.
Aluminum and its alloys
Aluminum is a non-ferrous metal that has a silvery-white tint and melts at a temperature of 650°C. In the periodic table it corresponds to the symbol Al. This element ranks third in abundance among all rocks in the earth's crust (oxygen is in first place, silicon is in second place). Under atmospheric conditions, an oxide film forms on the surface of aluminum, preventing the occurrence of corrosion.
Important properties of aluminum:
- Low density - only 2.7 g/cm3 (for example, copper - 8.94 g/cm3).
- High electrical conductivity (37*106 S/m) and thermal conductivity (203.5 W/(m K)).
- Low strength in its pure form - 50 MPa.
- The structure of the crystal lattice is face-centered cubic.
The metal is easily processed by pressure. It is widely used in the electrical industry: electrical conductors are made from aluminum. In steel production it is used for deoxidation. Utensils are also made from aluminum, but they are not suitable for making pickles and storing fermented milk products - the element is unstable in alkaline and acidic environments. Some steel parts are coated with aluminum (aluminizing process) to improve their heat resistance. Due to its low strength, aluminum is practically not used in its pure form.
When marking aluminum, the letter A is used in combination with a number that indicates the metal content. For example, the A99 grade contains 99.95% aluminum, and the A99 grade contains 99.99%. There is also a special purity grade - A999, which contains 99.999% aluminum.
Wrought aluminum alloys
Wrought aluminum alloys are divided into hardenable and non-hardenable.
Strengthenable wrought aluminum alloys are duralumins (A-Cu-Mg system) and high-strength alloys (Al-Cu-Mg-Zn). High mechanical properties and low specific gravity allow these alloys to be widely used in mechanical engineering, especially in the manufacture of aircraft parts.
The main alloying elements for duralumin are magnesium and copper. These alloys are marked with the letter D with a number. D1 is used to make propeller blades, D16 is used for spars, frames, and aircraft skins, and D17 is used for fastening rivets.
High-strength alloys, in addition to aluminum, copper and magnesium, contain zinc. Designated by the letter B and a number, they are used for the manufacture of parts of complex configurations, helicopter blades, and highly loaded structures.
Non-hardening wrought aluminum alloys are alloys of aluminum with manganese (marked AMts1) and magnesium (AMg2 and AMg3). They are well processed by welding, drawing, rolling, hot and cold stamping. They are characterized by high ductility, but are not very durable. They are produced mainly in the form of sheets, which are used for the manufacture of products of complex shapes (rivets, frames, etc.).
Aluminum-based casting alloys
The most widely used casting alloys of aluminum and silicon, called silumins. They contain more than 4.5% silicon and are designated by the letters AK with a brand number. Silumins combine low specific gravity with high mechanical and casting properties. They are used for complex casting of auto, motorcycle and aircraft parts, as well as for the production of certain types of household appliances - meat grinders, heat exchangers, sanitary fittings, etc.
Brands of duralumin
Depending on the scope of use and the need to impart the required properties to the material, different components are added to duralumin.
To protect against rust, the metal is anodized - coated with special substances.
Duralumin grades are divided into:
- hardened in a natural environment, designated by the letter “T”;
- those who have undergone the artificial aging procedure are designated using the symbols “T1”;
- anodized, that is, treated with special varnishes - with the letter “A” in the name of the material.
Areas of application
The use of duralumin is quite diverse. Plates, rods, sheets and wire are made from it. These materials are used to make various parts.
Duralumin products are used in the following areas:
- Aviation technology. Light alloy is used in the manufacture of aircraft and the creation of bodies of other aircraft - airships or rockets. This component is used to make the casing, power elements, steering rod materials, etc.
- Construction sector. Pipes, angles, sheets, etc. are often used in this area.
- Automotive industry. Bodies, radiators, and other components are made from duralumin substance.
- Production of drills. Drills, circles, etc. are made from metal.
- Dural is often used in everyday life; it is used to make foil used in baking or when wrapping sweets.
Areas of use
Copper alloys play a key role in meeting modern social needs - in the production of renewable energy, healthcare, high-efficiency energy devices, and communications. Here are some examples:
In the manufacture of ventilation, heating and air conditioning systems, copper alloys help reduce the labor intensity of manufacturing and assembling air conditioners, reduce their weight, reduce their size, increase the efficiency of the devices, and reduce refrigerant consumption.
In construction and architecture, copper alloys improve the appearance and expressiveness of buildings, and increase their resistance to floods and flooding. The use of copper alloys meets the important requirements of modern building design, which require the use of recyclable and environmentally friendly materials, thereby effectively protecting the environment.
In the electric power industry, copper alloys are used, ranging from the production technology of high-voltage wires and microcircuits to powerful generators and computers. Their role in matters of optimal distribution and generation of energy, including from renewable sources, is increasing.
The efficiency of using copper-based alloys increases with the introduction of recycling processes for substandard devices that contain parts made from these materials in their design.
Properties and characteristics
Due to the beneficial qualities of duralumin, it is used in manufacturing, in the manufacture of parts, and insulation.
Interesting: Metal cutting technology with gas
Characteristics are divided into the following types:
Physical and mechanical
The properties of duralumin are the lightness of the metal and resistance to high temperatures. In addition, the material has increased hardness. The density of duralumin is 2.8 g/m³, for steel this parameter is 8 g/m³. The melting point of duralumin alloy is +500 °C. A negative feature of the material is its susceptibility to corrosion as a result of exposure to high temperatures or heavy load.
Technological
A characteristic property of the metal is its ease of manufacture. The material can be made even at home: in the garage, etc. It does not need to be heated to extreme temperatures. Due to the simple production technology, the alloy is inexpensive to manufacture.
Alloys based on copper and aluminum
Alloys based on copper and aluminum
All alloys based on copper and aluminum, although they have sufficient physical and mechanical strength, do not have the necessary corrosion resistance. Therefore, in orthopedic dentistry they find very limited use (for the manufacture of temporary appliances in maxillofacial orthopedics). Recently, they have been successfully replaced in this section of orthopedic dentistry with stainless steel.
Copper-aluminum alloy (aluminum bronze) consists of 90%. copper and 10% aluminum, golden yellow in color, does not change it when in the oral cavity, despite the oxidation that occurs. Melting point 1030°, hardness 50, tensile strength 40 kg/mm2, elongation 30%, Aluminum bronze is used in the form of wire in orthodontics and maxillofacial orthopedics.
Copper-zinc-nickel alloy - nickel brass. An alloy of copper with zinc, and sometimes with the addition of small quantities of other elements, is called brass. Thus, nickel silver consists of 60-65% copper, 18-23% zinc and 12-22% nickel. The alloy has high strength and toughness, high anti-corrosion properties, and can be easily processed under pressure. In the oral cavity it becomes covered with a thin oxide film.
Duralumin contains, in addition to aluminum, copper 4%, magnesium, manganese, silicon and iron, approximately 0.5% each. The alloy is soft, ductile and easily deformed; at room temperature it strengthens over time. For annealing, they resort to heating at 350-370°.
Tin alloys
In the manufacture of various designs of dentures, it is necessary to obtain metal forms, dies and counter-dies. For this purpose, alloys based on tin and lead are used. These alloys, in addition to having a low melting point (hence the name low-melting alloys), have a relative hardness that ensures the stability of the alloy during operation. These alloys do not shrink very much when cooled.
To reduce shrinkage and slightly increase hardness, up to 50% bismuth is introduced into tin and lead alloys.
These alloys are a mechanical mixture type alloy. They are produced industrially in the form of blocks weighing about 50 g.
When working with alloys, it should be remembered that overheating not only leads to combustion of the metal, but also increases shrinkage and makes the alloy brittle. In addition, the lead and bismuth contained in the alloy easily combine with gold and platinum, causing them to become brittle and crack.
When metal overheats, it releases cadmium vapors, which are toxic to the body.
Types of alloys
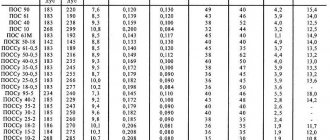
Taking into account the manufacturing method and exposure to different temperatures, the parameters of duralumin may change. There are the following types of metal:
- Aluminum with copper, magnesium, addition of manganese. Another name is “duralumin”. It is not hardened during creation. The compounds are used for the production of automobile radiators, hermetically sealed tanks, and pipes for the manufacture of gasoline pipelines. They are used to produce building materials. Alloys are easy to weld and do not rust easily. They are difficult to cut. However, additional coating must be used to protect against rust.
- Aluminum, magnesium or manganese. Another name is “mangaliya”. The material is complex in design. The main element is aluminum, other components are presented to give the alloy useful properties. Used for assembling space objects, aircraft, and high-speed trains. Slightly susceptible to corrosion, easy to weld. However, it does not tolerate exposure to a humid environment.
- Aluminum, magnesium and silicon. Another name for it is “avial”. It is well protected from corrosion and weighs little. It is used in high humidity and when electric current is passing through. During manufacturing, the alloy is hardened at a temperature of 525°C. Then it is sharply cooled with water to 20°C. The procedure lasts 10 days.
Interesting: What is surface hardening of steel
Magnesium and its alloys
Magnesium is a non-ferrous metal that has a silvery tint and is represented by the symbol Mg in the periodic table.
Important properties of magnesium:
- Melting point - 650°C.
- Density - 1.74 g/cm3.
- Hardness - 30-40 HB.
- Relative elongation - 6-17%.
- Tensile strength - 100-190 MPa.
The metal has high chemical activity and is not resistant to corrosion in atmospheric conditions. It cuts well, absorbs shock loads and dampens vibrations. Since magnesium has low mechanical properties, it is practically not used for structural purposes, but is used in pyrotechnics, the chemical industry and metallurgy. It often acts as a reducing agent, alloying element and deoxidizing agent in the manufacture of alloys.
When marking, the letters Mg are used with numbers that indicate the percentage of magnesium. For example, the Mg96 brand contains 99.96% magnesium, and Mg90 contains 99.9%.
Magnesium-based alloys are characterized by high specific strength (tensile strength - up to 400 MPa). They are well cut, ground, polished, forged, pressed, rolled. The disadvantages of magnesium alloys are low corrosion resistance, poor casting properties, and a tendency to ignite during manufacturing.
Wrought magnesium alloys
The most common are three groups of magnesium-based alloys.
Magnesium alloys alloyed with manganese
They contain up to 2.5% manganese and are not hardened by heat treatment. They have good corrosion resistance. Since these alloys are easy to weld, they are used for welded parts of simple configurations, as well as for fittings, oil and gasoline systems that do not experience heavy loads. Among this group are the MA1 and MA8 alloys.
Alloys of the Mg-Al-Zn-Mn system
The composition of these alloys, in addition to magnesium and manganese, includes aluminum and zinc. They significantly increase strength and ductility, making the alloys suitable for the manufacture of stamped and forged parts of complex shapes. This group includes brands MA2-1 and MA5.
Alloys of the Mg-Zn system
Alloys based on magnesium and zinc are additionally alloyed with cadmium, zirconium and rare earth metals. These are high-strength magnesium alloys that are used for parts subject to high loads (in airplanes, cars, machine tools, etc.). This group includes alloys of the MA14, MA15, MA19 grades.
Casting magnesium alloys
The most common group of cast magnesium alloys belongs to the Mg-Al-Zn system. These alloys practically do not absorb thermal neutrons, therefore they are widely used in nuclear technology. They are also used to make parts for airplanes, rockets, and cars (cabin doors, instrument housings, fuel tanks, etc.). Alloys of magnesium, zinc and aluminum are used in instrument making and in the manufacture of housings for electronic equipment. This group includes brands ML5 and ML6.
High-strength cast magnesium alloys have better mechanical and technological properties. They are used in aviation for the manufacture of loaded parts. This group includes alloys ML12 (magnesium, zinc and zirconium), ML8 (magnesium, zinc, zirconium and cadmium), ML9 (magnesium, zirconium, neodymium), ML10 (magnesium, zinc, zirconium, neodymium).
The difference between duralumin and aluminum
Dural and aluminum differ in chemical composition, which affects the specific application. In addition, duralumin has a specific gray color, and the source material has a light shade. However, the main difference between duralumin and aluminum is that it has no ductility, it is hard and fragile. The connection cannot be bent or made a dent. The chips made from it are brittle and brittle. Metal is easily scratched; if you look at the damage, it becomes clear that the material consists of small crystals.
There is an easy way to understand what material is in front of you. Drip sodium hydroxide onto the metal. If the stain darkens after 10 minutes, it is duralumin.
First alloys
The discovery of copper, as well as alloys containing this metal, occurred after accidental (and then intentional) heating of sulfide ores to a temperature of more than 8000C.
This process has been available to humanity since 4000-3000. BC, then copper alloys were obtained. Since the extraction of copper from copper ores occurred with the inevitable inclusion of associated chemical elements - silicon, tin, iron - in the composition of the final product, then in fact we were talking about obtaining bronze. Bronze is historically the first copper alloy. It is reliably known that bronze was already known in ancient Iran and the Balkans. Thus was born the metallurgy of the Bronze Age of mankind.
Much later, brass was discovered. Brass (later called “fake gold” for its dull yellow luster) was first received by the Romans during the reign of Emperor Octavian Augustus (beginning of our era). To do this, copper was fused with ore containing a large percentage of zinc.
Subsequently, the metallurgy of copper alloys was constantly improved: the amount of foreign impurities decreased, the accuracy of the composition of alloys containing copper increased, and their nomenclature grew.