A light gray metal with very high hardness, refractoriness and heaviness is tungsten. In addition to this, it has very high chemical resistance.
- Lighting
- Place of Birth
Tungsten ore mining
The tungsten content in the earth's crust is just over one ten-thousandth of a percent, which makes it a fairly rare natural resource. It is not found in its pure form, so minerals such as wolframite and scheelite are used to extract it. These are tungsten ores that contain, in addition to the base metal, a number of impurities.
In the mines
The underground method of mining ores containing tungsten involves the sequential collapse of horizontal layers of the mine with further accumulation of material in spent blocks (the so-called “magazine”). Then the collected workings are loaded onto transport and removed to the surface.
In the quarries
In them, the extraction of tungsten ores is carried out by open-pit mining. By collapsing external soil, loading it onto transport systems and sending it for processing.
Properties
Tungsten is a light gray metal with a characteristic luster, reminiscent of platinum. The peculiarity of this element is its refractoriness. It is worth noting a number of other important properties.
Physical
Thus, the metal has the highest melting point of 3420 °C and boiling point of 5555 °C. It is also characterized by the following:
- the density of tungsten without impurities is 19.25 g/cm³;
- has increased resistance to changes in vacuum;
- Brinell hardness rating is expressed as 488 kg/mm²;
- high thermal and electrical conductivity;
- paramagnetic;
- can be forged at a temperature of 1.6 thousand degrees Celsius.
The metal is deservedly recognized as one of the heaviest and hardest.
Optical
As for the optical characteristics, tungsten is considered isotropic according to the accepted type. When considered by pleochroism, it does not pleochroize. It is also non-fluorescent.
Crystallographic
The metal belongs to the space category Im3m with cubic system and hexaoctahedral point group.
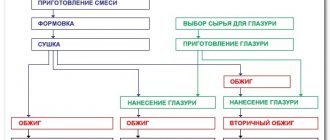
Tungsten production processes
Since fossil minerals contain a sufficient amount of impurities, to obtain tungsten itself it is necessary to use a three-stage technology:
- Enrichment of mined ores to form a solution or precipitate of the required concentration. This process includes gravity, flotation, magnetic or electrostatic separation. The result is the production of 60% tungsten anhydrite concentrate WO3.
- A chemical compound of high purity is obtained through a reduction reaction under the influence of hydrogen or carbon. This is often enough to obtain tungsten powder.
- But in order to produce compact solid ingots - bars, which are more convenient for further processing, pressing and sintering are used. In order for them to be easily forged, they are subjected to high temperatures.
However, that's not all. To obtain such popular products as metal rods, tungsten is processed at a temperature of about 15000C on a rotary forging machine.
To produce wire from the above-mentioned rods, they are drawn, first heated to 10,000 C, and then gradually cooled to 4,000 C. After which the finished wire is annealed, polished and electrolytically etched.
Obtaining monolithic metal
If the first two stages of industrial production of tungsten are well known to metallurgists and have been used for a very long time, then the development of a special technology was required to obtain a monolith from powder. Most metals are obtained by simple melting and then cast into molds, but with tungsten, due to its main property - refractoriness - such a procedure is impossible. The method of producing compact tungsten from powder, proposed at the beginning of the 20th century by the American Coolidge, is still used with various variations in our time. The essence of the method is that the powder turns into a monolithic metal under the influence of electric current. Instead of conventional smelting, several steps must be taken to obtain tungsten metal. At the first of them, the powder is pressed into special bars. Then these posts are subjected to a sintering procedure, and this is done in two stages:
- First, at temperatures up to 1300ºC, the rod is pre-sintered to increase its strength. The procedure is carried out in a special sealed oven with a continuous supply of hydrogen. Hydrogen is used for additional reduction; it penetrates into the porous structure of the material, and with additional exposure to high temperature, a purely metallic contact is created between the crystals of the sintered rod. After this stage, the headstock is significantly strengthened, losing up to 5% in size.
- Then proceed to the main stage - welding. This process is carried out at temperatures up to 3 thousandºC. The post is secured with clamping contacts, and an electric current is passed through it. Hydrogen is also used at this stage - it is needed to prevent oxidation. The current used is very high; for bars with a cross-section of 10x10 mm, a current of about 2500 A is required, and for a cross-section of 25x25 mm - about 9000 A. The voltage used is relatively small, from 10 to 20 V. For each batch of monolithic metal, a test bar is first welded, it is used to calibrate the welding mode. The duration of welding depends on the size of the post and usually ranges from 15 minutes to an hour. This stage, like the first, also leads to a reduction in the size of the stack.
The density and grain size of the resulting metal depend on the initial grain size of the rod and the maximum welding temperature. The loss of dimensions after two stages of sintering is up to 18% along the length. The final density is 17–18.5 g/cm².
To obtain highly purified tungsten, various additives are used that evaporate during the welding process, for example, oxides of silicon and alkali metals. As they heat up, these additives evaporate, taking other impurities with them. This process promotes additional cleaning. When using the correct temperature conditions and the absence of traces of moisture in a hydrogen atmosphere during sintering with the help of such additives, the degree of tungsten purification can be increased to 99.995%.
Tungsten connections
The most common tungsten compounds are its oxides, chlorides, and carbides.
Tungsten oxide, containing two oxygen atoms, is a dark brown crystal. Tri-oxygen tungsten is a lemon-colored powder.
Tungsten
Tungsten carbides - compounds of tungsten with carbon - have found very wide application in a number of industries due to their hardness. These are, first of all, composite materials and hard alloys such as Pobedit, as well as mixtures of carbides: tungsten, tantalum and titanium.
An alloy of tungsten and rhenium is used in the manufacture of thermocouples, which allow measuring temperatures above 20,000C. True, in chemically non-aggressive environments.
Tungsten sulfide is used as a high-temperature lubricant.
Some tungsten compounds are used as pigment dyes and catalysts for chemical reactions. Tungstic acid is used as an adsorbent and catalyst in the production of gasoline. Tungstate single crystals control flows of ionizing radiation, so in demand in medicine and nuclear physics.
Storage and transportation
The conditions for storage and transportation of powdered tungsten and products containing it in its composition (stubs, plates, rods, wire, electrodes) are determined by the requirements of the relevant state standards and technical specifications, which are reflected in the documentation for the manufactured products.
Since tungsten concentrate is non-toxic, explosion-proof and does not pose a fire hazard, its storage and transportation do not pose any significant difficulty. The only problem is its possibility of dust formation and the need to protect products from external mechanical influences and aggressive environments.
Therefore, tungsten powder must be packaged in specialized containers or double bags weighing no more than 50 kg, the outer layer of which must be made of synthetic fabric or polypropylene, the inner layer of paper or polyethylene. For long-term storage, the bags are formed into transport bags. The concentrate is transported in open rolling stock, and stored in packaged form in closed warehouses.
Tungsten electrodes for storage and transportation are packaged in cardboard boxes with foam or thick paper supports. The boxes are then placed in wooden crates protected with waterproof paper and further sealed with cotton wool or paper. Electrodes, unlike concentrate, must be transported in covered transport.
Similar protection measures are used for the preservation and movement of other products made of this metal.
Processed products
Due to its unique properties, primarily hardness and refractoriness, tungsten has found a wide range of applications since its discovery. As a refractory material, it is widely used in metallurgy. Although other industries cannot do without such valuable material.
Lighting
Due to their low electrical conductivity and low evaporation rate, at one time tungsten filaments made it possible to make a technical revolution in the entire industry of creating electric lighting devices, and also began to be used in the manufacture of electronic vacuum devices.
Shells
The high level of density of this material, reaching up to 19.3 g/cm3, along with its strength, provided gunsmiths with an excellent means of destroying armor. Today, tungsten is one of the main chemical elements that make up the heavy alloy cores of armor-piercing bullets and shells.
Tungsten scrap
Electrodes
Non-consumable tungsten electrodes are used as a welding material for a process carried out using gases. Helium or argon protects the connection point from atmospheric influences, and the electrode at this time withstands significant temperatures and a long service life. This allows you to create optimal working conditions while avoiding unnecessary costs.
History of discovery
It was first obtained by the Spanish de Elguiar brothers in 1783. The most interesting thing is that the name “tungsten” existed even before the discovery of the element itself. Back in the 16th century, metallurgists involved in tin mining observed that large amounts of tin were being lost, and this ore received this name.
In 1781, Scheele obtained tungsten trioxide from a mineral called scheelite. Then Scheele's discovery was proven by Bergman, who dug it up with a heavy stone.
However, Berzelius preferred another name - Tungsten. Tungsten could be called by different names: thistle, tungsten, scheelite or tungsten beetle.
In today's world, namely in the USA, France and Great Britain, tungsten is called tungsten.
Being in nature
Place of Birth
The geological structure of the earth's crust is such that the largest deposits of tungsten ores are located in the Alps, Himalayas, and mountain ranges of the Pacific Ocean region. These are the territories of Kazakhstan (the largest deposit is Upper Kairakty), China (the most productive deposit is Jianshi), Canada (Tangsten deposit) and the USA (significant reserves have been explored in the Climax deposit).
There are also areas of concentration of wolframites and scheelites in Bolivia, Portugal, Great Britain, Turkey, Russia, Uzbekistan, South Korea, and Australia.
In space
Progress does not stand still, and the earth's resources are distributed extremely unevenly and are quite limited. The exploration of outer space, which has made it possible to take samples from the surfaces of a number of celestial bodies of nearby objects in the Solar System, gives every reason to assume the presence of a huge amount of minerals on asteroids, comets and planets.
This opens up very attractive prospects for their future development. It is assumed that asteroids contain a huge amount of minerals, and in very high concentrations. Including tungsten. Due to the fact that some of these celestial bodies rotate close to the Earth, the prospects for their exploration become very, very tempting.
Governments of a number of countries, international space communities and private agencies are actively creating a legal framework, developing programs, and sending missions. Thus, Luxembourg was the first to pass a law allowing private mining in space. Not only the leading space powers of the world are active in this issue, but also Japan, India, Australia, and Israel. Active research is being carried out on the surface of the Moon, Mars, and Venus.
It is difficult to give any assessment to these efforts, since many organizational, technical and financial problems stand in the way. Although many experts consider it possible to mine tungsten in space in the 21st century.
Traps dry on the shore
Last week it became known that Russian Deputy Prime Minister Victoria Abramchenko instructed Rosrybolovstvo to hold auctions until April 15, 2022 to distribute the remaining quotas for catching deep-sea hard-to-catch crab species in the Far Eastern fishery basin. Previously, this specific resource was put up for auction several times, but to no avail due to a lack of bids due to the high price.
We are talking about quotas for deep-sea crab species - red snow crab and angulatus snow crab. Fishing for these crabs is difficult to access, since they are caught at depths of 800-1500 meters, and the fishing areas are remote from the coast. Working in such conditions places special demands on fishing vessels in terms of their autonomy; they must be equipped with more powerful and energy-intensive fishing equipment. Thus, catching deep-sea crab species is more expensive and less profitable compared to other crab species.
Ex-governor of Primorye Darkin loses his fishing business on Sakhalin Three of his companies have filed for bankruptcy
Crab auctions were held as part of the implementation of 50% of the crab catch quota provided for investment purposes. A total of 41 lots were put up for auction. In October 2022, 35 lots were sold, and 142.4 billion rubles were allocated to the budget.
Unallocated quotas were put up for new auctions three times, all to no avail due to a lack of applications. In September 2022, a decision was made to reduce the price of the lot. This made it possible to implement some of the quotas, but not all.
The piquancy of the situation is that perhaps the only contender for these crabs is JSC Fishing Collective Farm Vostok-1 (Primorsky Territory), one of the largest crab producers in the Russian Federation.
Last July, Vostok-1 went all-in, announcing that it was forced to stop fishing for deep-sea crabs due to the lack of quotas, which were not distributed during investment auctions in October 2022 and were not returned to industrial development in 2022.
The very act of stopping fishing due to the lack of quotas and removing vessels from fishing interrupts the planned influx of financial resources, and this fact already puts the enterprise itself on the brink of bankruptcy,” said the collective farm’s appeal addressed to the Prime Minister of the Russian Federation Mikhail Mishustin.
Of course, such a monster as Vostok-1 still needs to manage to go bankrupt, but the company is unlikely to want to work at a loss.
World reserves
The world's confirmed reserves of tungsten amount to 2.6 million tons. Identified resources amount to 12.5 million tons. Inferred resources are estimated at 9.5 million tons. Over 60 countries around the world have deposits of this metal:
- China – 7.5 million tons.
- Kazakhstan – 3.1 million tons.
- Russia – 3 million tons.
- Canada – 1.7 million tons.
- USA – 0.8 million tons.
- Australia – 0.7 million tons.
- Bolivia – 0.5 million tons.
It should be noted that a number of countries in the world community have deposits that are unsuitable for development due to their unprofitability. While the five leading ones have more than 70% of developed reserves in their territories.
Tungsten Mining Countries
The absolute leader in the production and export of tungsten on the world market is China. The share of this state is 82.7% (70 thousand tons) according to 2022 data. They produce significantly less:
- Vietnam – 4.8 thousand tons.
- Mongolia – 1.9 thousand tons.
- Russia – 1.5 thousand tons.
- Bolivia – 1.2 thousand tons.
It is obvious that European countries have lost this segment of the metals market to their Asian competitors, as their production volumes decreased sharply in 2019. Austria, Portugal and Spain together produced 2.14 thousand tons in 2022, and the UK completely stopped production, satisfying its demands by importing metal.
Author: Yuri Florinskikh All articles by this author
Latest articles by the author: The largest producers of milk and dairy products in the world Diamonds: properties, mining methods and applications
Tungsten is a chemical element with atomic number 74 in the periodic table, designated by the symbol W (Latin Wolframium), a solid gray transition metal. Tungsten is the most refractory of metals. Only the non-metallic element, carbon, has a higher melting point. Under standard conditions it is chemically resistant. The tungsten Clarke of the earth's crust is (according to Vinogradov) 1.3 g/t (0.0013% of the content in the earth's crust). Its average content in rocks, g/t: ultrabasic - 0.1, basic - 0.7, intermediate - 1.2, acidic - 1.9. Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO3 with oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. Wolframite (iron and manganese tungstate nFeWO4 * mMnWO4 - ferberite and hübnerite, respectively) and scheelite (calcium tungstate CaWO4) are of industrial importance. Tungsten minerals are usually disseminated into granitic rocks, so the average tungsten concentration is 1-2%. Kazakhstan, China, Canada and the USA have the largest reserves; deposits are also known in Bolivia, Portugal, Russia and South Korea. World tungsten production is 49-50 thousand tons per year, including 41 in China, 3.5 in Russia; Kazakhstan 0.7, Austria 0.5. Main exporters of tungsten: China, South Korea, Austria. Main importers: USA, Japan, Germany, UK. There are also tungsten deposits in Armenia and other countries.
Reserves at tungsten deposits in 2012, thousand tons *
| China | 1,900.0 |
| Russia | 250.0 |
| Canada | 120.0 |
| Bolivia | 53.0 |
| Austria | 10.0 |
| Other countries | 867.0 |
| Total stocks | 3,200.0 |
*data from US Geological Survey
Global tungsten supplies have struggled to keep up with rising demand in recent years, and primary tungsten production has been supplemented by sales of the metal from inventories, especially those held by government agencies (e.g., China, Russia, and the United States). Additionally, a period of low tungsten prices in the 1990s and early 2000s led to the exit of most Western manufacturers from the market, leaving Chinese companies to dominate tungsten production. The market's unhealthy confidence in Chinese tungsten has continued despite rising prices in recent years. The Chinese government has taken many measures to consolidate and manage the domestic tungsten industry and limit the production and export of most tungsten products. Despite pending WTO action regarding China's exports of many products, including tungsten, it seems unlikely to stop or reverse the Chinese government's tungsten plans. China is now also the largest consumer of tungsten, accounting for more than 50% of global demand, according to ITIA. This country is also the fastest growing market. As a result, China already needs and will continue to need more and more of its tungsten products to meet domestic demand, and if anything, tungsten exports (especially intermediate products) will be further constrained in the future. All of this poses a threat and an opportunity for the tungsten sector outside of China. Opportunities to increase production of tungsten ores, concentrates, and intermediates have been identified as supplies from China are likely to become more constrained. On the other hand, China's primacy in increasing the value of tungsten resources through the export of more processed forms of tungsten, as well as the production and export of goods made from tungsten, pose a real threat to those Western companies involved in such operations. Supply from current production facilities in the tungsten industry is unlikely to grow significantly in the coming years. In China, although there are many resources underground, any new operations or complete expansions of existing producers are more likely only to replace the development of open-pit mines, which are being depleted, with mines. So the days when China could turn on the signal and flood the market with cheap tungsten are long gone. In addition, most decisions regarding future tungsten production will now be made by the Chinese government with an eye on domestic demand. Of the existing large producers outside China, few, if any, are willing or able to significantly increase production. Cantung's Canadian facility, the largest mine-based tungsten producer outside of China, is already running at full capacity and has less than 3 years of proven ore reserves. In Portugal, the Beralt mine has very little room for significant expansion, and the Mittersil mine in Austria produces tungsten primarily for domestic use and is therefore unlikely to supply much metal to the free market. New projects are the clear way forward for the tungsten market to secure future supplies of the material and there are many promising candidates around the world. However, despite the increase in tungsten prices and the obvious needs of the tungsten market, the development of new projects is progressing very slowly. The credit crisis and subsequent economic downturn in 2009, accompanied by the current economic difficulties in the Eurozone, affected investor confidence and it was very difficult to do anything outside of financing. In addition, the lack of knowledge regarding the tungsten industry in the financial sector and the fact that tungsten production volumes are a “critical” factor, as is the case with rare earth metals, have also hampered the progress of tungsten projects. There are now signs that this is beginning to change and at government level tungsten is now regarded as a critical/strategic material. The EU has categorized tungsten as a "critical raw material", while the British Geological Survey has placed tungsten at the top of its list of "supply risk" materials. There seems to be more understanding that relying on supplies from China is not prudent, so tungsten projects should be encouraged. Previously, the US, EU and Japan filed a WTO complaint against China's export restrictions on certain critical materials, including tungsten. However, it is unlikely that this will lead to China exporting more tungsten, and if consumers outside China want to secure future supplies, designing and developing new mines outside of China would be a better bet. Many developments suggest that progress on some major tungsten projects has accelerated. For example, Wolf Minerals has received loan approval for £55 million from three banks, meaning the company now has almost three quarters of £110 million to start production. At the Sangdong project in South Korea, Woulfe Mining recently announced it had signed an agreement for US$104 million in debt financing, while a subsidiary of Berkshire Hathaway Inc. Warren Buffett announced that it had acquired a 25% stake in the project. Northcliff Resources Ltd. has acquired full ownership of the Sissons tungsten project in Canada from Geodex, while Masana Resources is accelerating development of the Nui Phao project in Vietnam. EMC Metals is working to restart the Springer tungsten mine in the US, and Ormonde Mining is pursuing its Barruecopardo project in Spain. Despite all the developments in various tungsten projects, significant tungsten tonnages from new sources are unlikely until 2013 at the earliest. Further funding delays and other problems could delay the launch of new projects until 2014 and beyond. Despite restrictions on the export of ores and intermediate products, China is still the main supplier of tungsten and tungsten products to the rest of the world and is likely to remain so for the foreseeable future. However, there is a continuing change in emphasis and, while supplies of ores, concentrates, and tungstates may come from new projects, China will further move down the value chain and look to export more processed forms of tungsten and increasingly finished products that contain tungsten. Until recently, the main tungsten trade flows were from China to large developed countries such as the US, EU and Japan. Now, in fact, China is a significant importer of tungsten ores and concentrates with an annual import volume of approximately 10,000 tons (gross weight). The main exporters of tungsten ores and concentrates are currently Russia, Canada, Portugal and Bolivia, while the USA and Germany are the main importing countries, excluding China. For tungstates, exports come from China, the Netherlands and the US with the main destinations being Germany, the US and Japan. Ferrotungsten is exported from China, the Netherlands, Germany and Brazil, mainly to the Netherlands, Japan, Germany, Austria and the USA. Finally, the main trade flows for tungsten metal (including waste and waste) are from China, Germany, the US, UK and South Korea to Germany, the US, UK and Japan. China is gradually reducing its tungsten export quota. In 2012, the share was set at 15,400 tons, which was 300 tons less than 15,700 tons in 2011, which in turn was 300 tons less than in 2010. In July, Chinese authorities released a second list of quotas (for the second half of 2012) for many metals, including tungsten. The second list concerns the 40% share of total exports during the year. The first batch, which covered 60% of the share, was announced in December 2011. In the second list, the export quota for tungsten and products was 7,587 tons, compared to 11,380 tons in the first list. To highlight the difficult conditions in the tungsten market in 2012, it should be noted that Chinese trade in tungsten and tungsten products declined in the first quarter of 2012. According to official customs data, imports of tungsten concentrates into China fell just over 8% in the first three months of 2012, compared with the same period in 2011 - 2,384 tons. Exports of tungsten trioxide from China fell 38% in the same three-month period. while tungsten carbide exports decreased by only 7%. At the same time, in monetary terms, Chinese trade in tungsten products increased, reflecting higher tungsten prices compared to the previous year.
Tungsten production in the world, tons*
* US Geological Survey data
Demand for tungsten has historically correlated very closely with the overall economic situation in the world, especially since tungsten is primarily used in traditional industries rather than in faster-growing new technologies. In recent years, the increase in tungsten consumption has outpaced global GDP growth, due to the influence of China (and its high economic growth rates). China currently accounts for approximately 50% of global tungsten demand, according to ITIA, and therefore has a commensurate influence on global consumption. Along with most metals, demand for tungsten has suffered from recessionary conditions brought on first by the credit crisis in late 2008 and 2009 and, more recently, by the Eurozone crisis. In 2009, global tungsten consumption fell by more than 25% from its 2007 peak, before returning to pre-crisis levels in 2010 and 2011. Preliminary data suggests that tungsten demand in 2012 was affected by problems in the Eurozone and contractionary conditions in most European economies. Global demand may have increased slightly in 2012, although it is likely to have remained the same as the previous year or even decreased slightly. The table below for 2011-2012 presents preliminary data on the tungsten market from various sources (CM Group, AMJ and others). The fact is that many European economies are in crisis and even growth in Germany, which is the largest economy in the region, is unlikely to be enough to pull the GDP growth of the entire regional economy to a positive value. This is likely to have a major impact on tungsten demand as Europe is still a significant market for tungsten. Europe's share of global metal consumption was around 30% in the past decade, but this has declined in recent years and with lower economic growth and ongoing challenges, Europe now accounts for less than 15% of global demand. However, difficulties in Europe are a concern for the tungsten industry as a whole because, while demand for tungsten in Europe may decline, European consumption of products that use tungsten (eg cutting tools, drills, light bulbs, etc.) , should decrease equally. There are also fears that the problems plaguing European countries could spread to other major industrialized economies, and that the Eurozone crisis could contaminate the global market. China and other BRICS countries have been the engine of global economic growth in the past few years, and have been a favorable factor for tungsten consumption. However, there are signs that growth in these countries is slowing, and it may be that the tungsten industry cannot rely on them to compensate for slow or negative growth in other areas. Growth in India, for example, fell from double digits to 5.4% in 2012; China's economic growth was 7.8% in 2012, down from an average of 10% between 2007 and 2011.
Tungsten consumption in the world, tons*
| year | 2008 | 2009 | 2010 | 2011 | 2012 |
| USA | 9250.0 | 7100.0 | 9300.0 | 11400.0 | 12000.0 |
| Europe | 12050.0 | 5850.0 | 8800.0 | 13500.0 | 12000.0 |
| Japan | 7750.0 | 2500.0 | 7350.0 | 5850.0 | 6000.0 |
| China | 31800.0 | 34350.0 | 39400.0 | 39500.0 | 40000.0 |
| Other countries | 4350.0 | 2200.0 | 6150.0 | 4750.0 | 5000.0 |
| Total | 65200.0 | 52000.0 | 71000.0 | 75000.0 | 75000.0 |
*data from International Tungsten Industry Association
Tungsten prices have risen faster than other similar metals over the past three to four years, illustrating the relatively strong fundamentals in the tungsten market. However, existing problems in the Eurozone have affected economic growth in Europe, and tungsten prices have also followed this crisis. During 2012, metal prices fell by approximately 10%, compared with a 15% drop in nickel prices over the same period. Fears that the Eurozone crisis would "pollute" the global economy appear to have only partially been realized in the tungsten industry, as Chinese export prices fell by only 5% during 2012. Interestingly, however, domestic prices in China have fallen by almost 20% over the same period, reflecting insufficient demand from the domestic Chinese tungsten industry and, conversely, the expansion of the global market. The global crisis in 2009 resulted in a 20% fall in prices, although most of this decline occurred in the first half of the year, before prices stabilized in the second half of 2009 on a brighter outlook for global economic activity. The structural strength of the tungsten market was illustrated by the smaller decline in tungsten prices compared to similar metals such as cobalt, molybdenum and nickel. While tungsten prices fell approximately 18% during 2009, cobalt prices fell 54%, molybdenum 59% and nickel 31% (although nickel prices had already fallen 43% in 2008). Since the credit crisis and economic downturn in 2009, the recovery in tungsten prices has also been faster and stronger than that of similar metals. This is the main clue to the current density of tungsten supply to the market. Supply constraints may be eased in the coming years by many potential tungsten projects that are at various stages of development around the world. Outside of China, significant tungsten projects are located in Australia, Canada, South Korea, the UK and Vietnam. Chinese tungsten prices are obviously a major barometer of global market levels, as it is a major producer and supplier of most forms of tungsten from intermediates such as trioxide to more processed products such as tungsten carbide and tungsten metal powders. One product that China no longer supplies to the global market is tungsten concentrate, although domestic concentrate prices provide a good indicator. Prices for tungsten concentrate in China, as well as oxide and carbide, followed trends in the global economy in 2012. Overall, prices have fallen between 10% for tungsten oxides, and 20% for tungsten concentrate. Tungsten carbide prices in China fell by about 15% over the same period. There was a slight increase in tungsten prices in May 2012, but insufficient demand during the summer lull meant prices continued their slow decline between June and August. Tungsten concentrate prices in China remain subdued due to weak demand from mills, which in turn are suffering from a lack of refining demand for tungsten. The short-term future for tungsten prices in China is a further downward drift as there appears to be a lack of refining demand affecting the oil and gas exploration and production industry. World tungsten prices have fallen less than Chinese prices. Tungsten prices should return to an upward trend in the coming years if demand strengthens in 2013, as supply from new projects is unlikely to impact the market until late 2013 at the earliest.
Tungsten prices, dollars/t WO3
The outlook for the tungsten market is relatively positive as demand is expected to grow at an average annual rate of almost 6% until 2016, driven by economic growth in China. Tungsten supplies will struggle to keep up with rising demand until at least 2013, when some potential tungsten-producing projects are expected to begin production. However, any delays in the commissioning of these projects will quickly create a growing shortage in the market with resulting upward pressure on prices. Very few new tungsten projects are expected to begin shipping in 2013, so the market will rely on existing producers to meet growing demand. As a result, there may be an increase in the shortage of metal on the market and an increase in the price to US$475 per ton in the near future. Tungsten prices are expected to decline by 2015, when most of the planned new tungsten projects come to market.