The grade book of steels and alloys contains information on the classification, application, chemical composition, physical and mechanical properties, weldability, technological parameters and information on the corrosion resistance of steels and alloys.
The stamp was created by specialists from Lasmet LLC. Our organization has the ability to manufacture most of the steels and alloys described in the brand book. You can place an order for the products you are interested in.
In the steel and alloys brand directory you can also find information about GOST standards for steel and download them. Also, when you hover over the symbols of physical quantities, you will receive a brief information about them.
If you did not find the answer to your question in the brand book, you can ask it here to the specialists of Lasmet LLC.
Review
The following table provides an overview of the various iron-nickel alloys. Naturally occurring alloys are a variety of minerals and called native elements or native metals. Some entries have more than one crystal structure (for example, meteoritic iron is a mixture of two crystal structures).
| Name | Description | Chemical Formula/Mass Percent Ni |
| Antitenitis | An intermetallic compound found in meteorites | Fe3Ni[4] |
| Avaruite | Native intermetallic compounds found in serpentinites and meteorites | Ni2Fe to Ni3Fe |
| Earth's core | The Earth's core is made of an alloy of iron and nickel. | about 5.5%[5] |
| Elinvar | The elasticity of this alloy does not change with temperature. | 36% (also 5% chromium) |
| Invar | Steel with very low thermal expansion | 36% |
| Kamacite | Native alloy found in meteorite iron | Fe0.9Ni0.1 |
| Maraging steel | Strong, malleable steel option | From 15 to 25% |
| Meteoric iron | The combination is mainly kamacitis and taenitis and a small amount of tetrataenitis, antitenitis and awaruite | 5–30% |
| Mu-metal | High Permeability Alloy | 77% |
| Planetary core | Planets, moons and planetesimals may have an iron-nickel alloy core. | Different |
| Tenit | Native alloy found in meteorites | NiFe |
| Telluric iron | Native alloy found on Earth (not extraterrestrial) | Fe (but 0.05 to 4% nickel) |
| Tetrataenitis | Native alloy found in meteorites | FeNi |
Nickel Applications
Let's figure out which industries the material is used in:
- Stainless steel production.
- Creation of protective coatings by galvanization.
- Application in powder metallurgy.
- Creation of alloys that do not contain iron.
- Production of chemical reagents.
This substance is used in the production of batteries. Nickel serves as a catalyst in industry. Alloying with titanium allows you to create good quality dental implants.
A nickel alloy containing from 20 to 60% iron is used in the production of cores for electromagnets.
Nickel is used to create:
- Foil.
- Wire.
- Pipes.
Nickel rolled products are used for interaction with alkalis in the chemical industry. Medical devices are made from this metal.
Chromel-Copel thermocouples are also used in industry. Nichromes are considered important metals for the manufacture of technically complex devices. The addition of silicon allows the creation of parts for electric heaters. The combination of nichrome, titanium and aluminum is called nimonics. The heat resistance of the materials allows them to be used in the design of aircraft engines. Casting alloys can be additionally alloyed to increase heat resistance. The structure of such metals is heterogeneous, so the properties of the substance do not coincide in certain areas.
Methods for manufacturing heat-resistant composite metals have been tested; the following elements are added to nickel:
- Thorium.
- Aluminum.
- Zirconium.
An alloy with the addition of thorium oxides is widely used. An increase in electrical resistance can be obtained after doping with such elements.
Use of nickel in pure form:
- The element is used to protect other connections from rust. Using electroforming, one substance is applied to another.
- Production of corrosion-resistant products that retain their original shape for a long time.
- Production of filters and catalysts used in industry.
- Creation of mechanical neutron beam interrupters.
Due to its high cost, nickel is only used in situations where iron or steel parts are not suitable. The substance is used for the manufacture of boilers, tanks for transporting and processing alkalis, storing chemical reagents, food, etc. Condensates are created in nickel pipes. Tools for working with aggressive substances are made from this material. Therefore, they are often used in medicine and when interacting with chemical reagents.
The material is used in nuclear physics; its properties make it possible to generate powerful neural impulses.
Nickel and iron-nickel alloys
At operating temperatures above 7000C, it becomes necessary to use heat-resistant alloys based on nickel or iron-nickel bases instead of steels. These alloys, which have both high heat resistance and heat resistance, contain in certain combinations a large number of alloying elements (nickel, cobalt, iron, titanium, chromium, zirconium, vanadium, niobium, molybdenum, tantalum, tungsten, rhenium, etc.) and are highly alloyed. The number of entered elements ranges from 10 to 15.
Nickel forms the basis of modern heat-resistant and heat-resistant alloys. Nickel is a silvery-white metal. Specific gravity 8.902 g/cm3, melting point 1453°C, has a face-centered cubic lattice. Ferromagnetic.
In its pure form it is very plastic and can be processed by pressure, has the following physical and mechanical properties:
- specific electrical resistance 0.0684 μOhm∙m;
- coefficient of linear thermal expansion α=13.5∙10−6 K−1 at 0 °C;
— coefficient of volumetric thermal expansion β=38—39∙10−6 K−1;
- elastic modulus 196–210 GPa.
Nickel is characterized by high corrosion resistance - it is stable in air, water, alkalis, and a number of acids. Chemical resistance is due to its tendency to passivation - the formation of a dense oxide film on its surface, which has a protective effect.
For nickel-based
These include alloys whose main structure is a solid solution of chromium and other alloying elements in a nickel base (nickel content of at least 50%).
For iron-nickel based
include alloys whose main structure is a solid solution of chromium and other alloying elements in an iron-nickel base (the sum of nickel and iron is more than 65% with an approximate ratio of nickel to iron of 1:1.5). Heat-resistant alloys (HS) of the Ni – Cr, Ni – Cr – Fe and Ni – Cr – Al – Ti, Ni – Cr – Al – Co systems are the most important groups of nickel-based alloys. ZhS alloys are widely used in modern gas turbine aircraft engines and ground-based gas turbine units: turbine blades and disks, guide vanes and combustion chambers are made from them. These alloys are also widely used in units of the metallurgical industry, boiler making, internal combustion engines, equipment in the petrochemical and oil refining industries.
Most nickel alloys are polycrystalline. Creep deformation in such materials develops due to the movement of dislocations in grains, grain boundary sliding and diffusion movement.
The movement of dislocations at temperatures above 0.3 Tm occurs in two ways - sliding and creep. When heated, the accumulation of atoms of alloying elements and impurities around the dislocation dissolves, and this facilitates sliding.
Climbing dislocations
is ensured by their interaction with vacancies, due to which individual sections of dislocations are displaced into separate regions of the crystal. Heating accelerates the diffusion influx of vacancies and facilitates creep.
Grain boundary sliding
represents a shift of grains relative to each other along common boundaries in a narrow boundary region. Slip develops under the influence of tangential stresses. The smaller the grain, the greater the sliding deformation.
Diffusion transfer
associated with the movement of vacancies along the boundaries and inside grains. Under the influence of tensile stresses, the energy of vacancy formation decreases. At the boundary between two stretched grains, the concentration of vacancies increases and they move to zones where their concentration is lower. The flow of vacancies corresponds to a counter flow of atoms, therefore, at stretched boundaries, the number of atoms increases and the grains elongate. The transfer of atoms also occurs throughout the volume of grains, however, the contribution of volume diffusion is insignificant and plays a role only at high temperatures of about 0.9 Tmel, and the effect of grain boundary diffusion is significant already at 0.4-0.6 Tmel.
Polycrystalline
Nickel alloys are operated at temperatures of about 850 °C, which is slightly more than 0.7 of the melting point of the alloy (1280 °C).
Further increases in operating temperatures are achieved by directional crystallization
. Such nickel alloys have operating temperatures that are 0.9 times the melting point. For the manufacture of working and nozzle blades, casting alloys ZhS6K, VZHL-12U are used at an operating temperature of 950-10000, ZhS6UVI, ZhS6F, ZhS26VI, ZhS30, ZhS32, etc. at 1050-11000.
Alloying elements in nickel alloys can be divided into several categories:
— elements that form solid solutions with nickel, strengthening the matrix g-phase (Co, Cr, Mo, W, Fe);
— elements that form intermetallic compounds with nickel -g'-phase (Al, Ti, Mb, Ta, Hf);
— elements forming carbide compounds (Cr, Mo, W, Nb, Ta, Ti); -elements that improve casting properties, weldability, deformability, etc. (C, V,);
— elements that refine the alloy and contribute to the formation of a fine-grained structure (B, Zr, Hf, Y, rare earth elements).
The main phases present in nickel alloys are:
-g-phase
, has a face-centered cubic lattice. Usually it is a solid solution with elements such as chromium, molybdenum, tungsten;
-g'-phase
- the main strengthening phase of nickel alloys of the Ni3(Al, Ti) type, coherently associated with the g-phase. Interestingly, the yield strength of the g'-phase increases with increasing temperature, starting from 650 °C. The g'-phase is quite viscous, which gives the alloy heat resistance and strength without embrittlement of the material. As the volume fraction of the g'-phase increases, the heat resistance increases. In modern alloys, the proportion of the g'-phase reaches 70%. There are many factors that determine the strengthening effect of the g'-phase: the size of its particles, the content of alloying elements, volume fraction, etc.
Carbide phases.
The carbon content in nickel alloys is 0.05 ... 0.2%. When it interacts with carbide-forming elements, carbides of the TiC, TaC or HfC type are formed. Subsequent heat treatment converts these parent carbides into forms with lower carbon content, such as Me23C6 and Me6C. They tend to precipitate at grain boundaries, resulting in increased tensile strength at high temperatures.
Topological close-packed phases.
These include the s-phase, m-phase, and Laves phases. These phases, unlike g', precipitate in the form of plates. On metallographic sections they are visible as needles. Lamellar-shaped phases negatively affect the mechanical properties of nickel alloys, in particular toughness and creep resistance, and contribute to the initiation of cracks due to their brittleness.
Depending on the area of application, nickel-based structural alloys are divided into two groups:
- deformable
alloys intended for use in corrosive environments and at high temperatures are supplied in accordance with GOST 5632;
— foundries
Nickel-based alloys intended for the manufacture of gas turbine engine parts, supplied according to specifications.
Based on their structure, nickel alloys are divided into homogeneous (nichromes) and heterogeneous (nimonics).
Nichromes.
The basis of these alloys is nickel, and the main alloying element is chromium (ХН60У, ХН78Т). Nichromes do not have high heat resistance, but they are very heat resistant. They are used for lightly loaded parts operating in oxidizing environments, including heating elements.
Nimoniki
are nickel-based alloys. They include chromium (about 20%), titanium (about 2%), aluminum (about 1%). Examples of alloys: KhN77TYu (EI437A), KhN77TYUR (EI437B), KhN70MVTYUB, KhN55VMTFKYu. These alloys are used only in a heat-treated state. Heat treatment consists of hardening from 1050...1150oC in air and aging at 600...800oC.
When marking nickel-based alloys, the same alphanumeric system is used as when marking steels. The letter X at the beginning of the mark means that the alloy contains chromium. Its percentage is not indicated in the brand - it makes up the remainder up to 100%. For example, the alloy KhN77TYu contains about 77% nickel (the basis of the alloy), about 2.5% titanium and about 0.8% aluminum.
By using complex alloying of nimonics, it is possible to obtain higher heat-resistant properties or ensure reliable operation at higher temperatures.
Very small impurities of lead, tin or antimony have a detrimental effect on the heat resistance of nickel-based alloys. They are located predominantly along the grain boundaries and weaken the adhesion forces between them at high temperatures. A harmful impurity is sulfur, which with nickel forms NiS sulfide, which has a low melting point. Sulfide with nickel forms a eutectic with a melting point of 6250C. At the same time, technological ductility sharply deteriorates during hot pressure treatment. Welded joints become prone to cracking. It is desirable that the sulfur content does not exceed 0.005-0.010%.
Heat treatment of alloys
is largely determined by the chosen alloying system and usually consists of hardening and aging.
The quenching temperature is selected from the condition of obtaining a homogeneous solid solution. For example, the KhN50VMTYUB alloy is subjected to quenching in air from a temperature of 11400C and subsequent aging at a temperature of 9000C for 5 hours, and the KhN68VMTYUK alloy is quenched from a temperature of 11000C followed by aging at a temperature of 9000C for 5 hours. During aging, dispersed particles are released from the supersaturated solid solution strengthening particles g'
-phases and alloys are strengthened.
Availability g'
-phase increases heat resistance and at the same time imparts to alloys a tendency to form hot cracks during welding and heat treatment, the need for heat treatment of parts after welding or welding for technological and operational defects.
The properties of heat-resistant nickel alloys for blades and disks of gas turbines are determined by the thermal stability of the structure, size, shape and amount of strengthening g'
-phase, strength characteristics of
the g
-solid solution, the optimal ratio of crystal lattice parameters of the
g'-
and
g
-phases, distribution of the carbide phase and other factors.
Heat-resistant weldable nickel-based alloys
developed at VIAM and have found wide application for combustion and combustion chambers, screens, nozzles, combustion chamber and HPC housings, rear turbine supports, high-temperature gas ducts, etc. For modern and future aircraft gas turbine engines, new materials have been developed that make it possible to increase the operating temperatures of combustion chambers by 150 -200°C (table 4.1.)
The introduction of cobalt has a positive effect on the strength, ductility and toughness of the austenite matrix. This was used in the development of the VZh145 (EK102) alloy (15% W, 30% Co, 20% Cr). The alloy is structurally stable and significantly exceeds commercial alloys for similar purposes in terms of strength, heat resistance and heat resistance. It is technologically advanced and welds well with all types of welding.
A feature of the new alloy VZh159 is its high ductility and manufacturability characteristics (at the level of the properties of the homogeneous alloy EI868), while short-term strength properties and long-term strength in the operating temperature range from 650 to 1000°C are also at a high level (Table 4.1). It is also very important that the alloy significantly exceeds in heat resistance all serial homogeneous alloys based on Ni-Cr and Ni-Cr-Co bases: for example, under a test mode of 1000°C ↔ 200°C, the new alloy can withstand 500 cycles (the heat resistance of the serial alloy EP648 is 75 cycles).
The chemical composition of the VZh159 alloy does not contain the scarce alloying elements Co and W (alloy density 8250 kg/m3), the material welds well.
For high-temperature components of combustion chambers and other parts of the hot section of gas turbine engines, a new alloy VZh155 with an operating temperature of up to 1200°C has been developed (see Table 4.1). Such heat resistance properties were obtained as a result of the development of a special composition based on Ni-Cr and an original regime of chemical-thermal treatment. The high heat resistance of the VZh155 alloy is ensured by a structure containing stable dispersed nitride precipitates. These precipitates have high thermal stability and are more stable at high temperatures than intermetallic compounds.
Table 4.1. Properties of new heat-resistant weldable alloys
for combustion chambers of advanced gas turbine engines
| Alloy | ,MPa | , MPa, at temperature, °C | ||
| VZh145(EK102) | 850-900 | |||
| VZh152* | — | — | ||
| VZh159 | 170-180 | — | 20-25 | — |
| VZh155 | — | — |
For welded highly loaded stator assemblies and parts (combustion chamber housing, rear support, nozzle apparatus, afterburner combustion chamber, etc.) operating at temperatures of 600-950°C, a whole series of heat-resistant alloys has been developed, which have found application in 5th generation gas turbine engines .
To ensure modern parameters for weight transfer, hull materials must have high strength at room and operating temperatures, high heat resistance, satisfactory weldability with all types of welding (argon arc welding, resistance spot welding, etc.), good manufacturability when carrying out hot and cold metal forming. Currently, the most widely used for these purposes is the iron-nickel-based heat-resistant alloy EP718-ID (Table 4.2).
Table 4.2. Properties of heat-resistant alloys for welded housings
| Alloy | KCU, J/cm2 | , MPa, at temperature, °C | ||||
| MPa | % | |||||
| EP718 | 780-810 | 320-360 | ||||
| VZh151 | 20-30 | ― | 800-850 | ― | ||
| VZh159 | 1100-1250 | 600-650 | 35-40 | ― | 600* | ― |
| VZh168 | 27-30 | ― | ― | ― | ||
| VZh169 | 24-28 | ― | ― |
*At 650°C.
For the combustion chamber housings of promising gas turbine engines, a high-strength alloy VZh151 has been developed, intended for long-term operation at temperatures of 700-750°C, sparingly alloyed with scarce elements (W, Mo) and therefore having a reduced density (8370 kg/m3). Welded joints of this alloy are characterized by high corrosion resistance in all climatic conditions and are not prone to intergranular corrosion and stress-corrosion cracking.
Control questions
1. Name the features of the thermophysical properties of nickel.
2. Why do nickel alloys have high heat resistance?
3. What is the difference between nickel and iron-nickel base alloys?
4. What groups are nickel alloys divided into according to their intended purpose?
5. Describe the properties of nimonics. Name the areas of their application.
6. Describe the properties of nichromes. Name their scope.
7. Name the mechanisms of heat resistance of alloys.
8. Due to what alloying mechanisms is the heat resistance of nickel alloys ensured?
9. Characterize the properties of the g¢-phase in nickel alloys?
10. What heat-resistant nickel alloys that can be welded do you know?
Copper alloys
Pure copper is a reddish-pink metal, crystallizes in the lattice of a face-centered cube, and has no allotropic transformations. The melting point of copper is 1083°C. The density of copper is 8.94 g/cm3. In terms of electrical and thermal conductivity, copper ranks second after silver. The electrical resistivity of copper is 0.018 Ohm • mm2/m, and the thermal conductivity coefficient at 20 °C is 385 W/(m • K).
Copper has high corrosion resistance in fresh and sea water, atmospheric conditions, organic acids, caustic environments
Copper and its alloys are widely used in the construction of power lines and various types of communications, in electrical engineering and instrument making, in refrigeration technology (production of heat exchangers for cooling devices) and chemical engineering (production of vacuum devices, coils). About 50% of all copper is consumed by the electrical industry
Depending on the purity, copper is produced in the following grades: M00, M0, M1, M2, M3. The mechanical properties of copper depend on its condition and the amount of impurities. Impurities sharply reduce heat and electrical conductivity, ductility and corrosion resistance. These include Fe, P, As, Si, Pb. Cold plastic deformation increases strength but reduces ductility and electrical conductivity. To remove hardening, annealing is carried out at a temperature of 550-650°C. Copper is rolled into sheets, pipes, tape, and wire. It is easy to polish, solder and weld. At the same time, it is poorly processed by cutting and has low fluidity.
While retaining the positive properties of copper, copper alloys have good mechanical technological and antifriction properties. According to their technological properties, copper alloys are divided into wrought and cast alloys.
.
According to their ability to be hardened - into those that can be hardened and those that cannot be hardened by heat treatment.
Based on their chemical composition, they are divided into two main groups:
brass and bronze
.
Brass.
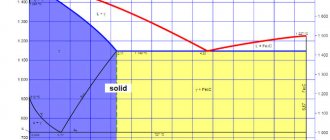
Brasses are dual or multicomponent copper-based alloys in which the main alloying element is zinc. According to the Cu-Zn phase diagram (Figure 5.1.), copper and zinc form a solid a-solution with a maximum solubility of zinc of 39%. With a higher zinc content, the electronic compound CuZn (b-phase) is formed. In practice, the b-phase appears in the structure of brass already at a zinc content of 30%.
The mechanical properties of brass also change in accordance with the structure. If brass has an a-solid solution structure, an increase in the zinc content leads to an increase in its strength and ductility. The appearance of the b-phase is accompanied by a sharp decrease in plasticity. The strength continues to increase up to a zinc content of 45%, but the transition of brass to a single-phase state (b-phase) is accompanied by a sharp decrease in strength.
Figure 5.1.- State diagram of copper-zinc alloys
Therefore, in practice, brasses containing up to 30% zinc are used. Double brass is marked with the letter “L” and a number indicating the copper content in percent (L96, L90, L80). In brands of multi-component brass, additional letters and numbers are entered indicating the name and quantity of the component as a percentage (LAN 59-3-2) - i.e. 59% Cu, 3% Al, 2% Ni, the rest Zn 36%. Double brasses are divided according to their structure into two groups with the a-solid solution structure and two-phase brasses with the a+b structure. Single-phase brass with a zinc content of up to 30% (L90, L80) has the best ductility; higher strength and hardness - two-phase brass (L68, L63, L60).
Increasing the zinc content reduces the cost of brass, improves its machinability, ability to break in and resist wear. At the same time, heat and electrical conductivity decrease, and corrosion resistance deteriorates. Alloys containing more than 20% zinc are prone to corrosion cracking.
Cold-rolled semi-finished products in the form of strips, wires, and sheets are made from single-phase brass.
Due to their low ductility, two-phase brass is subjected to hot rolling; pipes, sheets, rods, and stampings are made.
Double brasses, despite their good fluidity, are not used for shaped casting since they have a rather large concentrated shrinkage cavity.
Multicomponent brasses are used in the form of deformable semi-finished products and shaped castings. Common alloying elements include aluminum, tin, silicon, and nickel.
Aluminum increases strength, hardness and corrosion resistance, so brass (LA 77-2) is well-formed and resistant to seawater.
Tin increases corrosion resistance in sea water, which is why brasses LO70-1, LO62-1 are called marine brasses.
Silicon increases the corrosion resistance and technological properties of brass. The fluidity, weldability, and deformability of the alloy (for example, LK80-3) improves. Nickel improves the mechanical properties and increases the corrosion resistance of brass.
Bronze
called alloys of copper with all elements except zinc. Bronzes are divided into tin, aluminum, beryllium, and silicon.
Bronze is marked with the letters “B” followed by letters and then numbers indicating the name and percentage content of alloy components (for example, Br OTSS 5-5-5 contains 5% tin, 5% zinc, 5% lead, 85% copper).
Tin bronzes.
From the state diagram of copper-tin alloys it follows that the limiting solubility of tin corresponds to 15.8% (Figure 5.2.).
Figure 5.2. State diagram of copper-tin alloys
In real conditions, as a result of the tendency of these alloys to nonequilibrium crystallization, the zone of the a-solid solution narrows and a eutectoid (a + b) appears, where the b-phase is an electronic compound Cu31Sn8 with a complex cubic lattice. The appearance of the b-phase leads to a sharp drop in ductility, so bronzes containing up to 14% tin are of practical importance. Double bronzes have poor fluidity and are prone to dendritic segregation and diffuse shrinkage porosity. Tin bronzes are alloyed with zinc, lead, nickel, and phosphorus.
Zinc improves fluidity, density of castings, mechanical properties, welding and soldering ability.
Lead improves the anti-friction properties and machinability of tin bronzes.
Phosphorus is a deoxidizing agent and at the same time increases the fluidity, wear resistance, and antifriction properties of bronzes.
Tin bronzes have high corrosion and chemical resistance. Bronzes are widely used for steam-water fittings operating under pressure. They are well processed by cutting, but are worse welded. Among copper alloys, tin bronzes have the lowest casting shrinkage (1%) and are therefore used to produce complex shaped castings.
Aluminum bronzes.
These are bronze grades
BrA9Ts2L, BrA10Kh4NCHL, BrA9Zh4N4MTs1. Aluminum bronzes are distinguished by high mechanical, anti-corrosion and anti-friction properties.
However, they have a large shrinkage during crystallization, which is why it is sometimes difficult to obtain a shaped casting. Increasing the aluminum content to 4-5% preserves the single-phase structure of the alloy, and this leads to increased strength and ductility. A further increase in aluminum content to 10-11% leads to a decrease in ductility and an increase in strength, because the structure becomes two-phase: a+b, where b is the CuAl electronic compound. Two-phase bronzes can be hardened. The disadvantages of aluminum bronzes are the tendency to gas saturation and oxidation during melting, and the formation of a coarse, coarse-grained structure. These disadvantages are significantly reduced when bronze is alloyed with iron, nickel, and manganese. Beryllium bronzes.
They are characterized by very high strength, elasticity, hardness, corrosion resistance combined with increased resistance to fatigue, creep, and wear. Double beryllium bronzes contain about 2% beryllium. Beryllium bronzes are subjected to hardening heat treatment - hardening and artificial aging. These bronzes are heat-resistant materials. They operate stably at temperatures of 300-350 °C. Beryllium bronzes are used to produce deformable semi-finished products and shaped castings. Beryllium bronze is used to make parts that operate at high speeds and pressures, and tools that do not produce sparks. The main disadvantage of these bronzes is their very high cost.
Silicon bronzes.
Silicon bronzes include bronzes of the
BrKn1-3, BrKMts3-1 . They have good elastic and antifriction properties, and high corrosion resistance.
They are substitutes for expensive tin and beryllium bronzes. They are well processed and welded. Casting properties are lower than those of other bronzes. Additions of manganese and nickel increase casting properties, strength and elasticity. These bronzes can be hardened and artificially aged. Silicon bronzes are used to replace tin bronzes for antifriction parts and beryllium bronzes in the production of spring parts. They are processed by pressure and are rarely used for castings. Control questions
1. What is the melting point of copper.
2. What is the density of copper?
3. What are the thermal and electrical conductivities of copper?
4. Name the grades of copper. On what basis are they supplied?
5. What is the corrosion resistance of copper and its alloys?
6. Give a classification of copper alloys by application.
7. What is the composition of brass? Decipher the brand of brass L90?
8. What alloying elements can be included in the composition of brass? Decipher the brass brandLAN 59-3-2.
9.How is brass classified depending on its structure?
10. Which brasses have increased ductility and why?
11. Which brasses have increased strength and hardness and why?
12. What is the composition of the so-called “marine” brasses?
13. What is the composition of bronzes? Decipher the bronze grade Br OTSS 5-5-5.
14. What properties does beryllium give to bronze?
15. What properties does silicon impart to bronze?
16. What is the composition of the b-phase in bronze and what properties does it impart to bronze?
Test questions for all sections
Nickel parameters
The color of the material is silver-white, the melting point is 1453 degrees. Nickel alloys are characterized by ductility and are well processed under pressure. The material evaporates at a temperature of 2732 degrees. Chemical properties allow the formation of compounds with different levels of oxidation. Under normal conditions, a thin oxide layer appears on the material. Nickel and alloys made from it are resistant to rust, are easily affected by nitric acid, and do not interact with other concentrated liquids.
A chemical reaction is possible with the following substances:
- Inert gases.
- Potassium.
- Cesium.
- Strontium.
- Iridium.
Carbon additives make it possible to obtain carbonyl, from which the purest materials can be created. When interacting with oxygen, nickel powder explodes.