To give steel special qualities, special impurities are used, which are called alloying elements. They are introduced into the alloy composition during the smelting process when certain conditions are created. Nickel, chromium, titanium, cobalt, molybdenum, aluminum and others are used as such substances. As a result, chromium-nickel, manganese, cobalt, titanium steels and the like are obtained. For carbon steels, manganese and silicon are mainly used, since it is these components in the required proportions that impart the desired properties to such alloys.
Description of pearlitic steels
Pearlite is formed by fairly slow cooling in the iron-carbon system at the eutectoid point on the Fe-C phase diagram (723°C, eutectoid temperature). In a pure Fe-C alloy, it contains about 88 volume percent ferrite and 12 volume percent cementite. Pearlite is known for its viscosity, and in a highly deformed state, for its very high strength.
When examined under a microscope, pearlite has a characteristic appearance created by thin lamellar stripes. It resembles nacre, a natural plate-like structure found in some species of molluscs. However, it does not follow from this that perlite is created through the natural deposition of successive layers. It is formed as a result of special processing of the eutectoid mixture, separating the hardness and strength indicators.
Pearlite is a product of the decomposition of austenite as a result of a eutectoid reaction, therefore all steels of the class under consideration are characterized by a lamellar arrangement of ferrite and cementite. Pearlite grows as nodules at the boundaries of preexisting austenite, so each nodule may have a different colony or orientation. These nodules may propagate to cover the preceding austenite boundaries. By changing the reaction temperature, the distance or length scale of any pearlitic grade steel can be changed by cementite branching.
Features of special steels and ways to obtain them
To give steel special qualities, special impurities are used, which are called alloying elements. They are introduced into the alloy composition during the smelting process when certain conditions are created.
Nickel, chromium, titanium, cobalt, molybdenum, aluminum and others are used as such substances. As a result, chromium-nickel, manganese, cobalt, titanium steels and the like are obtained.
For carbon steels, manganese and silicon are mainly used, since it is these components in the required proportions that impart the desired properties to such alloys.
Classification
The main parameters for classifying special steels is their structure. For such materials, the critical points are shifted downward, and therefore, when slowly cooled in air, they can acquire additional qualities. Based on this, they were divided into four classes.
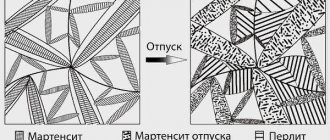
Martensitic steels
The structure of such materials is needle-shaped and consists of martensite, which implies a carbon content of at least 0.15%, chromium of about 11-17% and a number of additional components in the form of vanadium, nickel, tungsten, molybdenum.
It predominates in many pure and hardened metals. In this case, the martensitic component includes a carbon solution of iron in the form of a crystal lattice, which has a nonequilibrium structure.
This is why martensitic steels have significant internal stress. These materials include alloys under the following brands:
- 20Х13 – contains 12-14% chromium, up to 1% manganese and silicon, 0.16-0.25% carbon (nickel alloying is not possible);
- 10Х12НДЛ – characterized by a high nickel content (up to 1.5%);
- 18Х11МНФБ – composition includes molybdenum up to 1.1%, chromium 11.5%, carbon 0.8%, nickel 1%;
- 10Kh9MFB, 12Kh11V2MF, 13Kh11N2V2MF and 15Kh11MF are alloyed with molybdenum and vanadium in different proportions.
All of these materials are characterized by high hardness, corrosion resistance, heat resistance, the ability to self-harden, hydrogen resistance and low ductility. But with such indicators they are quite fragile. In this regard, cutting and welding them is quite difficult.
Pearlitic steels
Such special types of steels are classified as low or medium alloy steels. They contain pearlite and ferrite. Moreover, both components are alloyed with chromium. As a result, the material is highly resistant to cold brittleness.
In addition, the cooling rate affects the initial qualities of the alloy. When it changes, pearlite can acquire various transition structures. But a lot depends on what alloying impurities the steel contains. Some may help improve strength, toughness and heat sensitivity.
Pearlitic steels include 12МХ, 15ХМ, 12Х1МФ, 20ХМ, 25Х1МФ, 25Х2М1Ф, 18Х3МВ, 20Х3МВФ. All materials can be hardened, but at different temperatures.
Austenitic steels
Alloys of this nature are distinguished by the fact that they have the largest amount of impurities. As a result, they retain the austenite structure at any cooling rate. To strengthen them, they do not resort to heat treatment.
However, they may have different characteristics. With a chromium content of 12-18%, corrosion resistance increases, and with 17-25%, cold resistance increases. Also, with the help of impurities, you can change the heat resistance and heat resistance indicators.
In general, austenitic steels have high toughness, good density and high resistance to mechanical stress. Among the negative aspects, it is worth highlighting the difficulty of processing with a cutter.
The list of special alloys of this class is quite extensive, since it includes high-nickel, manganese, chromium-nickel, chromium-nickel-manganese, metastable and other alloys.
Carbide steels
Carbide class alloys contain significant amounts of carbon, chromium, molybdenum, tungsten and vanadium. All these components contribute to the formation of a strong austenitic matrix and stable carbides.
During crystallization from the liquid state, which results in a decrease in the dissolution of carbon in austenite, ledeburite is formed in the alloy. It is capable of maintaining high hardness at significant temperatures, and therefore is widely used for the manufacture of tools for rapid cutting of various steels.
The most striking example of such steels is the material produced under the brand name R6M5. Also included in this class are chrome-tungsten, chrome-molybdenum, and high-chromium alloys.
Effect of impurities on steels
Various impurities can give metals the desired characteristics. So, carbon, manganese, chromium, and molybdenum are used to increase hardness. Nickel and vanadium help improve viscosity.
Manganese, silicon, and aluminum are used for shrinkage. Abrasion resistance is increased by manganese, nickel, and chlorine. Nickel, chromium, and copper provide excellent corrosion resistance. But it is important not only to combine the impurities correctly.
The final characteristics largely depend on their proportions.
For example, special manganese steels must contain at least 14% of the corresponding component. When this indicator deviates, the structure of the alloy changes:
- 0.4-0.6% – martensitic;
- 10% and 12% – austenitic;
- 0.5% and 3.5% – pearlite.
However, the chlorine content remains unchanged in all three cases. In general, Mn affects thermal conductivity, so heating and cooling of such materials should be carried out with extreme caution. Products from it are produced only by casting, since cutting is very difficult. But manganese steels are well processed under pressure and do not have magnetic properties.
Another example of special steels is chromium alloy. The corresponding component is a carbide-forming component, so no more than 1% Cr is added to some steels. Even with this content, an increase in critical points is inevitable, so it is necessary to harden the material at high temperatures.
1% Cr is also found in tool alloys. In this quantity, it increases hardness and cutting characteristics.
Recently, alloying of alloys is carried out not with one component, but with several at once. In this case, it is possible to increase the influence of impurities on steel and obtain materials with special qualities. These include:
- high-speed - do not lose hardness after heating;
- wear-resistant - resistant to mechanical wear, welded after heating;
- automatic - additionally alloyed with lead, calcium and selenium, have low strength;
- spring – characterized by good elasticity, viscosity and resilience;
- construction – characterized by hardness, impact strength and elongation.
This is not the entire list of special steels. There are a great variety of them, so it is better to learn more about the composition or characteristics of a particular material from the manufacturer.
February 15, 2017
with friends:
Characteristics and markings
- Typically, the pearlite structure is quantitatively characterized by three parameters.
- Percentage of ferrite and pearlite.
- The distance between the perlite plates.
- Diameter of perlite nodules.
These parameters vary depending on the transformation temperature. The conditions necessary to obtain a fully pearlitic structure by continuous cooling are determined for ordinary carbon steels, which contain from 0.2% to 0.8% carbon.
When the carbon content becomes less than 0.6%, pearlite is always degenerate: it has a low yield strength, but it has good ductility, in particular, an increased linear stretch coefficient.
Pearlitic steels containing more than 0.6% C always have normal cementite platelets with a high yield strength, but with a slight decrease in area. There is no special marking for pearlitic steels, since they are all low- or medium-carbon structural steels (alloyed or unalloyed). Therefore, the technical requirements for steels of this class are fully covered by GOST 1050-88 and GOST 4543-2016: these steels contain no more than 0.30...0.60% carbon, with a relatively small amount of alloying elements. Mainly chromium, molybdenum or nickel. Typical representatives are steels 20Х, 50ХН, 30 ХМ, etc.
Heat-resistant and heat-resistant steels and alloys.
HEAT-RESISTANT AND HEAT-RESISTANT STEEL AND ALLOYSSTRUCTURE AND MECHANICAL PROPERTIES AT ROOM AND HIGH TEMPERATURES (N.S. Samoilov)
Heat resistant
are called steels and alloys that retain high mechanical strength at elevated temperatures for a certain time and at the same time have sufficient heat resistance.
Heat-resistant (scale-resistant)
are called steels and alloys that are resistant to chemical destruction of the surface in gas environments at temperatures above 550 0 C, operating in an unloaded or lightly loaded state.
Heat resistance is characterized mainly by the limits of creep and long-term strength. Approximately, heat resistance is also judged by mechanical properties, determined by a short-term tensile test at operating temperature.
Additional characteristics of heat resistance: long-term ductility, relaxation resistance, endurance limit, heat resistance, etc.
The heat resistance of steel (alloy) is determined by the chemical composition and structure; elements that increase heat resistance include molybdenum, tungsten, vanadium, niobium, titanium, cobalt, aluminum and, to some extent, chromium and nickel. The latter, along with manganese, is important mainly as an austenite-forming element (since the austenitic structure creates the greatest heat resistance of steel). Chromium has less effect on heat-resistant properties than many other elements. However, its presence in steel or alloy along with aluminum and silicon increases their heat resistance (scale resistance). Therefore, chromium is an essential component of heat-resistant steels and alloys.
Classification
Heat-resistant steels include alloys based on iron if the iron content exceeds 50%.
Depending on the total content of alloying elements, heat-resistant steels can be low-, medium- and high-alloy.
In low-alloy steel, the total content of alloying elements does not exceed 4-5%. Medium alloy steel is a steel with a total content of alloying elements from 5 to 9%, and the content of each of them should not exceed 5%. High-alloy steel is called steel in which the content of any alloying element exceeds 5%, or the total content of all alloying elements is more than 10%.
Based on their microstructure (obtained after cooling in air at a high temperature), heat-resistant steels are divided into seven classes: pearlitic, martensitic, martensitic-ferritic, ferritic, austenitic-martensitic, austenitic-ferritic, austenitic.
Low-alloy steels belong to the pearlitic class, medium-alloy steels belong to the pearlitic, martensitic or martensitic-ferritic class, and high-alloy steels belong to any of the listed classes except pearlitic.
Iron-nickel alloys include alloys whose main structure is a solid solution of chromium and other alloying elements in an iron-nickel base. The total content of iron and nickel is not less than 65%.
Nickel-based alloys include alloys containing at least 50% Ni, the main structure of which is a solid solution of chromium and other alloying elements in nickel (iron content no more than 6-8%).
Pearlitic steels
Among low-alloy steels, molybdenum-containing steels are characterized by high heat resistance, for example, chromium-molybdenum, chromium-molybdenum-vanadium, chromium-molybdenum-tungsten-vanadium, which have fairly high creep resistance and long-term strength at temperatures up to 565-580 ° C. Such steels are conventionally called heat-resistant.
The chemical composition of heat-resistant pearlitic steels is given in GOST 20072-74, GOST 4543-71, TU 14-1-1391-75. They contain 0.5-3.3% Cr; 0.25-1.2% Mo; 0.15-0.8% V. Some brands contain 0.3-0.8% W or Nb.
These steels are used for the manufacture of various parts in the boiler industry, operating for a long time (10,000-100,000 hours) at temperatures of 500-580 ° C, in particular, for steam pipes and superheating pipes, as well as for rolled products and forgings used in turbines and steam high pressure boilers.
The mechanical properties of grade metal from pearlitic steels, provided for by GOST or existing specifications, as well as recommended heat treatment modes are given in Table. 1. Mechanical properties at elevated temperatures, determined by a short-term tensile test, are, as a rule, not regulated. Of decisive importance are the standards of long-term strength and creep at operating temperatures, depending on the service life over a period of 10,000-100,000 hours (Table 2). Information on the approximate purpose of pearlitic steels and their operating temperatures is given in table. 3.
Martensitic steels
Martensitic steels contain 4.5-12% Cr, as well as significantly smaller amounts of Ni, W, Mo, V.
Steel grades 15Х5, 15Х5М, 15Х5ВФ and 15Х8ВФ are widely used for the manufacture of equipment elements for oil refineries - parts of pumps, valves, fasteners, cracking pipes operating at temperatures of 550-600 ° C. Steels of the same group with a higher Cr content (6-10%) and a higher Si content (2-3%) are mainly used for the manufacture of valves for internal combustion engines.
Steel 11Х11Н2ВМФ (EI962) is used for compressor disks and other parts operating at temperatures up to 600 °C with a limited service life.
The mechanical characteristics of martensitic steels are given in table. 1 heat resistance characteristics - in table. 12.2.
Martensitic-ferritic steels
Steels of the martensitic-ferritic class contain 10-25% ferrite in their structure in addition to martensite. The main alloying additive in these steels is Cr (11-13%), along with which there are less significant additives Ni, W, Mo, Nb, V (modified chromium steels). Their heat treatment consists of either quenching and tempering, or normalizing and tempering. The mechanical properties at the proper tempering temperature are almost equivalent. The level of heat-resistant properties after optimal heat treatment for most steels of the martensitic-ferritic class is also approximately the same. However, the highest (when processed to the same hardness) heat resistance characteristics at 500-600 °C are for steel 18Kh12VMBFR (EI993).
These steels are produced in the form of rolled products and are used in turbine construction for turbine blades and disks, as well as for fasteners. The approximate operating temperature for steel 15Kh12VNMF(EI802) is 550-580 °C and 570-600 °C for steel 18Kh12VMBFR(EI993).
Austenitic steels
Austenitic steels are mainly chromium-nickel steels with a content of Cr and Ni ranging from 7 to 25% each, along with which there are W, Mo, Ti, Nb, etc.
This is the largest group of heat-resistant (and heat-resistant) steels (see GOST 5632-72).
Table 1
Heat treatment modes and characteristics of mechanical properties of long products made of heat-resistant steels at normal temperature
Steel
| Class | Heat treatment mode | Characteristics of mechanical properties | ||||||||
| Temperature of hardening or normalization, °C | Cooling medium | Temperature (or annealing), °C | Cooling medium | σв, MPa | σ0.2, MPa | δ5, % | ψ, % | KSU, J/cm2 | ||
| 12MH(12ХМ) | Pearlitic | 920 ± 10 | air | 680-690 | air | 420 | 260 | 21 | 45 | 60 |
| 15ХМ | 900-920 | air | 630-650 | — | 450 | 280 | 20 | 45 | 70 | |
| 12Х1МФ(12ХМФ,ЭИ575) | 960-980 | air | 740-760 | air | 480 | 260 | 21 | 55 | 100 | |
| 20ХМ | 860-880 | oil | 500-600 | air | 800 | 600 | 12 | 50 | 90 | |
| 25Х1МФ(ЭИ10) | 880-900 | oil | 640-660 | air | 900 | 750 | 14 | 50 | 60 | |
| 25Х2М1Ф(ЭИ723) | 1030-1060 | air | 680-720 | air | 900 | 750 | 10 | 40 | 30 | |
| 18Х3МВ(ЭИ578) | 960 ± 10 | oil | 660-680 | air | 650 | 450 | 18 | — | 120 | |
| 20Х3МВФ(ЭИ579) | 1030-1080 | oil | 660-700 | air | 900 | 750 | 12 | 40 | 80 | |
| 15Х5М | Martensitic | 950-980 | air | 860 ± 20 | air | 450 | 220 | 20 | 50 | 120 |
| 15Х5 | — | air | 850-870 | air | 400 | 170 | 24 | 50 | 100 | |
| 15Х5ВФ* | — | air | 850-870 | with oven | 400 | 220 | 22 | 50 | 120 | |
| 40Х9С2(4Х9С2,ЭСХ8)* | — | air | 850-870 | with oven | 750 | 500 | 15 | 35 | — | |
| 40Х10С2М(ЭИ107) | 1050 | air or oil | 750±30 | oil | 950 | 750 | 10 | 35 | > 20 | |
| 15Х11МФ | 1095 | oil | 710 | air | 755 | 568-755 | 14 | 50 | 59 | |
| 18Х11МNFБ(EP291) | 1080-1130 | air, oil | 660-770 | air | 740 | 590-735 | 15 | 50 | 59 | |
| 20Х12ВНМФ(EP428) | 1010-1060 | oil | 660-770 | air | 740 | 590-755 | 14 | 45 | 54 | |
| 30Х13Н7С2(ЭИ72) | 1050+800 | water, oil | 660-680 | air | 1200 | 800 | 18 | 25 | > 20 | |
| 11Х11Н2В2МФ | 1000-1020 | air or oil | 660-680 | air | 900 | 750 | 12 | 50 | 80 | |
| 16Х11Н2В2МФ(ЭИ962А) | 1000-1020 | Same | 550-590 | air | 1000 | 850 | 10 | 45 | 70 | |
| 20X13(EZh2) | 1000-1030 | Same | 680-720 | oil, air | 660 | 450 | 16 | 55 | 80 | |
| 13Х11Н2В2МФ-Ш(ЭИ961-Ш) | 1000-1020 | air, oil | 660-710 | air | 880 | 735 | 15 | 55 | 88 | |
| 12Х1 | Martensitic-ferritic | 1020-1050 | air or oil | 700-750 | oil | 600 | 420 | 20 | 60 | 90 |
| 15Х11МФ | 1030-1100 | air | 700-740 | oil | 700 | 500 | 15 | 55 | 120 | |
| 15Х12ВНМФ(ЭИ802) | 1000-1020 | air, oil | 540-590 | air | 1080 | 930 | 13 | 55 | 88 | |
| 15Х11ВНМФ | 1010-1060 | oil | 660-770 | air | 740 | 590-735 | 14 | 45 | 54 | |
| 18X12VMBFR(EI993) | 1050 | oil | 650-700 | air | 750 | 500 | 14 | 50 | 60 | |
| 18Х12ВМБФР-Ш(ЭИ993-Ш) | 1030-1050 | oil | 680-720 | air | 800 | 680 | 12 | 45 | 59 | |
| 15Х12В2МФ | 1050 | oil | 680 | air | 800 | 600 | 15 | 50 | 70 | |
| 20Х20Н14С2(DI911) | Austenitic-ferritic | 1000-1150 | air, water | — | — | 590 | 295 | 35 | 55 | — |
| 20Х23Н13(ЭИ319) | 1100-1150 | air, oil, water | — | — | 490 | 295 | 35 | 50 | — | |
* Steel is used in annealed condition
table 2
Heat treatment modes, creep limits and long-term strength of alloy steels of pearlitic and martensitic classes used for long service
| Steel | Class | Heat treatment mode | Test temperature, °C | Long-term strength limit, MPa per time, h | Creep limit, MPa, corresponding to 1% deformation over time, h | |||||
| Temperature of hardening or normalization, °C | Cooling medium | Temperature, °C | Cooling medium | 10 000 | 100 000 | 10 000 | 100 000 | |||
| 12MH(12ХМ) | Pearlitic | 920 | air | 680-690 | air | 480 | 250 | 200 | 220 | 150 |
| 510 | 160 | 120 | — | 700 | ||||||
| 540 | 110 | 70 | — | 38 | ||||||
| 12Х1МФ(12ХМФ,ЭИ575) | 960-980 | air | 740-760 | air | 520 | 200 | 160 | 180 | 130 | |
| 560 | 140 | 108 | 118 | 84 | ||||||
| 580 | 120 | 90-100 | 90 | 62 | ||||||
| 25Х1МФ(ЭИ10) | 880-900 | oil | 640-660 | water | 500 | 260-290 | — | — | 80 | |
| 550 | 100-150 | — | 90 | 30 | ||||||
| 25Х2М1Ф(ЭИ723) | 1050 | air | 680-700 | air | 550 | 180-220 | 140-480 | — | 70 | |
| 18Х3МВ(ЭИ578) | 900 ± 10 | oil | 660-680 | air | 450 | — | — | 230 | 160 | |
| 500 | — | — | 120 | — | ||||||
| 550 | — | — | 75 | — | ||||||
| 20Х3МВФ(ЭИ579) | 1030-1080 | oil | 660-700 | air | 500 | 340 | 300 | 180 | 150 | |
| 550 | 200 | 160 | 130 | 100 | ||||||
| 580 | 140 | 100 | — | 50 | ||||||
| 15Х5М | Martensitic and martensitic-ferritic, austenitic-ferritic | 950-980 | air | 860 ± 20 | air | 480 | 180 | 150 | 105 | 70 |
| 540 | 100 | 75 | 65 | 40 | ||||||
| 15Х5ВФ* | — | 860 ± 10 | 500 | 120 | 92 | 85 | 60 | |||
| 550 | 90 | 70 | 50 | 38 | ||||||
| 600 | 65 | 52 | 38 | 28 | ||||||
| 20Х12ВНМФ(EP428) | 1010-1060 | oil | 660-770 | air | 450 | — | — | — | 274 | |
| 500 | 382 | 343 | — | — | ||||||
| 600 | 103 | 88 | — | 54 | ||||||
| 12Х13 | 1030-1050 | oil | 730-750 | air | 470 | 260 | 220 | — | — | |
| 500 | 220 | 190 | — | 57 | ||||||
| 530 | 190 | 160 | — | — | ||||||
| 13Х11Н2В2МФ-Ш(ЭИ961-Ш) | 1000-1020 | air, oil | 660-710 | air | 500 | 392 | s100 = 568 | — | — | |
| 550 | — | s100 = 441 | — | — | ||||||
| 600 | — | s100 = 294 | — | — | ||||||
| 15Х12ВНМФ(ЭИ802) | 1000 | oil | 680 | air | 550 | 250 | 220 | — | 100 | |
| 565 | 240 | 200 | — | 80-90 | ||||||
| 580 | 190 | 160 | — | 70-80 | ||||||
| 600 | 140-160 | 120 | — | 50-60 | ||||||
| 15Х11МФ | 1050 | air | 740 | — | 550 | 200 | 130-150 | — | 90-100 | |
| 600 | 100 | — | — | 40-50 | ||||||
| 18X12VMBFR(EI993) | 1050 | oil | 650-700 | air | 560 | 250-300 | 220-260 | — | 150 | |
| 590 | 210-240 | 170-200 | — | 100 | ||||||
| 620 | 140 | 110 | — | 50 | ||||||
| 15Х12В2МФ | 1050 | oil | 680 | air | 575 | 170 | 150 | — | 75 | |
| 600 | 150 | 130 | — | 45 | ||||||
| 630 | 110 | 85 | — | — | ||||||
| 20Х20Н14С2(DI911) | 1000-1150 | air, water | — | — | 875 | — | — | 9,8 | — | |
| 1000 | — | — | 1,4 | — | ||||||
| 20Х23Н13(ЭИ319) | 1100-1150 | air, oil, water | — | — | 550 | 151 | 57 | — | — | |
- The steel is used in the annealed state
Table 3
Approximate purpose of low-alloy heat-resistant pearlitic steels
| Steel | Purpose | Operating temperature, ˚ C | Life time | Temperature of the beginning of intensive scale formation, ˚ C |
| 12MH(12ХМ) | Pipes of steam heaters, steam pipelines and manifolds of power plants; fittings for steam boilers and steam pipelines | 500-510 | Very long lasting | 570 |
| 15ХМ | 520-530 | 570 | ||
| 12Х1МФ(12ХМФ,ЭИ575) | 570-585 | 600 | ||
| 15Х1М1Ф | 570-585 | 600 | ||
| 18Х3МВ(ЭИ578) | Pipes for hydrogenation plants and petrochemical equipment | 450-500 | Long | 600 |
| 20Х3МВФ(ЭИ579) | 500-550 | 600 | ||
| 20Х3МВФ(ЭИ579) | Forgings (rotors, discs), bolts | 530-560 | 600 | |
| 25Х1МФ(ЭИ10) | Fasteners (bolts, studs), flat springs | 500-510 | Long | 600 |
| 25Х2М1Ф(ЭИ723) | 520-550 | 600 |
Table 4
Heat treatment modes and characteristics of the mechanical properties of long products made of heat-resistant austenitic steels (at normal temperature)
| Steel | Heat treatment mode | Characteristics of mechanical properties | ||||||
| Quenching temperature, °C. | Cooling medium | T , °C, duration of holiday or aging | Tensile strength σв, MPa | Yield strength σ0.2, MPa | Relative elongation δ5, % | Relative narrowing ψ, % | Impact strength KSU, J/cm2 | |
| 10Х11Н20Т3Р(ЭИ696) | 1150-1180 | air, oil | 750 (16 h) | 850 | 500 | 10 | 15 | 30 |
| 10Х11Н23Т3МР-ВД (ЭП33ВД) | 1170-1200 | air | 750 (16-25 h) | 900 | 600 | 8 | 10 | 30 |
| 37Х12Н8Г8МФБ-Ш (ЭИ-481Ш, 4Х12Н8Г8МФБ) | 1140-1160 | water | 670 (12-14 h) 770-800 (10-12 h) | 850 | 600 | 15 | 20 | — |
| 45Х14Н14В2М(ЭИ69) | ** | 820 | 720 | 320 | 20 | 35 | 50 | |
| 09Х14Н18В2Б | 1110-1140 | air | * | 500 | 200 | 35 | — | — |
| 09Х14Н19В2БР(ЭИ695) | 1100-1150 | air | * | 500 | 220 | 38 | 50 | 140 |
| 09X14N19V2BR1(EI695) | 1130-1160 | air | 750 | 520 | 220 | 30 | 44 | 120 |
| 37Х12Н8Г8МФБ-Ш (ЭИ-481Ш, 4Х12Н8Г8МФБ) | 1140 ± 10 | water | 770-800 | 850 | 600 | 15 | 20 | 25 |
| 30Х13Г18Ф | 1150 ± 10 | water | 700 (10 h) | 700 | 360 | 30 | 40 | 80 |
| 08Х16Н13М2Б(ЭИ680) | 1100-1150 | water, air | 750 | 560 | 220 | 40 | 50 | 120 |
| 10Х17Н13М2Т(ЭИ448) | 1050-1100 | water | * | 520 | 220 | 40 | 55 | — |
| 08Х17Н15М3Т(ЭИ580) | 1050-1100 | air | * | 500 | 200 | 35 | 45 | — |
| 08Х15Н24В4ТР(ЭП164) | 1130-1150 | air | 730-750 | 750 | 450 | 20 | 35 | 80 |
| 08Х15Н24В4ТР(ЭП164) | ** | air | 700 (16 h) | 700 | 400 | 15 | 30 | — |
| 12Х18Н9 | 1050-1100 | air, water | 700 (20 h) | 500 | 200 | 45 | 55 | — |
| 08Х18Н10Т(ЭИ914) | 1050-1100 | Same | 700 (20 h) | 520 | 200 | 40 | 55 | — |
| 12Х18Н9Т | 1050-1100 | Same | 700 (20 h) | 550 | 200 | 40 | 55 | — |
| 12Х18Н12Т | 1050-1100 | Same | 800 (10 h) | 550 | 200 | 40 | 55 | — |
| 08Х18Н12Б(ЭИ402) | 1050-1100 | Same | * | 500 | 180 | 40 | 55 | — |
| 36Х18Н25С2 | 1100-1150 | air, oil, water | * | 650 | 350 | 25 | 40 | — |
| 36Х18Н25С2 | 1200 | water | 800 (8 hours) | 855 | 550 | 17 | 18 | 50 |
| 30Х19Н9МВБТ | 1150-1180 | air, water | 750-800 | 680 | 350 | 35 | 40 | 60 |
| 31Х19Н9МВБТ(ЭИ572) | 1050 | water | 750 (15 h) | 680 | 350 | 25 | 25 | 70 |
| 55X20N4AG9M | 1160-1190 | water | 760-780 | 1000 | 650 | 8 | 10 | — |
| 20Х20Н14С2(DI911) | 1000-1100 | air, water | * | 600 | 300 | 35 | 30 | — |
| 20Х23Н13(ЭИ319) | 1050-1150 | Same | * | 500 | 300 | 35 | 50 | — |
| 20Х23Н18(EI-417) | 1100-1150 | Same | * | 500 | 200 | 35 | 50 | — |
| 20Х23Н18(EI-417) | 1030-1130 | water | * | 540 | 265 | 35 | — | — |
| 20Х25Н20С2(ЭИ283) | 1100-1150 | air, water | * | 600 | 300 | 35 | 50 | — |
*Available without vacation. **Without hardening
The following designations for alloying elements are accepted in the grades of these steels: A - N, B - Nb, B - W, G - Mn, K - Co, M - Mo, N - Ni, P - B, C - Si, T - Ti , F - V, X - Cr, Yu - Al. The number after the letter indicates the rounded (average) content of this element as a percentage (if the content is less than 1%, the number is not written). The exception is carbon, the content of which is expressed in tenths of a percent in the first two digits of the brand. For example, grade 45Х14Н14В2М(ЭИ69) has the following composition: 0.45% C, 14% Cr, 14% Ni, 2% W, and ≤ 1% Mo. The characteristics of the mechanical properties of long products made of heat-resistant austenitic steels, as well as optimal heat treatment modes, are given in Table. 4.
In accordance with the characteristics of alloyed austenite, the characteristics of the heat-resistant properties of austenitic steels are higher (Table 5) than those of heat-resistant steels of pearlitic or martensitic classes.
Steel 08Х18Н10Т(ЭИ914) is used as heat-resistant and heat-resistant. At temperatures up to 600 °C, steel has stable mechanical properties, it is resistant to intergranular corrosion and welds well. Steel of this grade is produced in the form of long products, forgings, sheets, pipes for power and chemical equipment. Steel 12Х18Н12Т has similar properties, which is used in the same fields of technology.
Chromium-nickel-tungsten austenitic steels (45Х14Н14В2М (ЭИ69)) have increased heat resistance and fatigue resistance at high temperatures. Steel 45Х14Н14В2М (ЭИ69) is used for exhaust valves of internal combustion engines. For long service life at temperatures of 600-650 °C, steel of the same type with a reduced C content (up to 0.15%) is recommended.
Austenitic steels are used, as a rule, for the manufacture of parts operating at temperatures of 650-700 °C for a very long time. The mechanical properties of these steels at a temperature of 20 °C are similar, but the limits of long-term strength and creep differ quite significantly (Tables 4, 5). The most heat-resistant of them are steel 09Х14Н19В2БР(ЭИ695)
and 09X14N19V2BR1(EI695)
, which are used for the manufacture of superheating and steam pipes for ultra-high pressure installations.
Chromium-manganese steels of grades 30Х13Г18Ф and 37Х12Н8Г8МФБ-Ш (EI-481Ш, 4Х12Н8Г8МФБ) are substitutes for heat-resistant steels with a higher nickel content. These steels have fairly high long-term strength at temperatures of 500-650 °C.
Table 5
Creep and long-term strength limits of heat-resistant austenitic steels used for long-term service*
| Steel | Temperature, °C | Long-term strength limit, MPa per time, h | Creep limit, MPa, corresponding to 1% deformation over time, h | ||
| 10 000 | 100 000 | 10 000 | 100 000 | ||
| 09Х14Н18В2Б | 600 | 180 | 140 | 120 | 110 |
| 650 | 140 | 110 | 105 | 85 | |
| 700 | 90 | 65 | 60 | 50 | |
| 09Х14Н19В2БР(ЭИ695) | 650 | 168 | 130 | 140 | 110 |
| 700 | 125 | 95 | 85 | 65 | |
| 750 | 70 | 55 | — | — | |
| 09X14N19V2BR1(EI695) | 600 | 260 | 230 | 250 | 170 |
| 650 | 215 | 190 | 200 | 140 | |
| 700 | 170 | 140 | 120 | 85-90 | |
| 12Х18Н10Т | 600 | 150 | 110 | — | 75 |
| 650 | 80-100 | — | — | 30-40 | |
| 30Х19Н9МВБТ | 600 | 240 | 220 | — | 110 |
| 650 | 170 | 150 | — | 80 | |
| 12Х18Н12Т | 600 | 170 | 135 | — | — |
| 650 | 105 | 75 | — | — | |
| 08Х16Н13М2Б(ЭИ680) | 600 | 200 | 150 | 140-170 | 90-120 |
| 650 | 130 | 60**-90 | 100-120 | 50-70 | |
| 700 | 60-70 | 30-50 | 60 | 20 | |
| 10Х17Н13М2Т(ЭИ448) | 550 | 280 | 240 | — | 110 |
| 600 | 180 | 130 | 110 | 60 | |
| 650 | 110 | 70 | 90 | 50 | |
| 700 | 40/80** | 30 | 55** | 28** | |
| 20Х20Н14С2(DI911) | 650 | — | — | 65 | — |
| 700 | — | — | 30 | — | |
| 800 | — | — | 10 | — | |
| 20Х23Н13(ЭИ319) | 550 | 240 | 200 | 150 | 60 |
| 600 | 190 | 150 | 70-80** | 50** | |
| 650 | 110 | 70 | 50-60** | 30** | |
| 700 | 60 | 36 | 30 | 14 | |
| 20Х23Н18(EI-417) | 600 | 150** | 100 | 90 | 60** |
| 650 | 110 | 60**-80 | 50-60 | 40**-54 | |
| 700 | 50**-60 | 35 | 35 | 28**-35 | |
| 800 | 21 | 12-21 | — | 7**-12 | |
| 20Х25Н20С2(ЭИ283) | Almost like steel 20Х23Н18 (EI-417) | ||||
* Heat treatment modes, see table. 4.
** Data from foreign sources for steels of similar chemical composition.
Iron-nickel alloys
Iron-nickel alloys can be divided into two groups: 1) containing 14-16% Cr and 32-38% Ni and 2) containing 20-25% Cr and 25-45% Ni (or Ni + Mn) . The alloys of the first group are additionally alloyed with tungsten and titanium and have high (approximately equal) heat resistance (Table 6). Alloys of the second group, due to the increased Cr content, are heat-resistant; in terms of heat-resistant properties, they are inferior to alloys of the first group, for example, alloy KhN38VT (EI703).
Alloys KhN35VT(EI612), KhN35VMT, KhN35VTYu(EI787) are supplied mainly in the form of hot-rolled and forged rods and strips, as well as forgings. Alloys KhN35V5T, KhN38VT (EI703) and 12Kh25N16G7AR (EI835) are mainly used to make hot-rolled and cold-rolled sheets and strips, and alloys KhN45Yu (EP747) are also used to make pipes. Basically, iron-nickel alloys are used for the manufacture of parts for steam and gas turbines.
Nickel-based alloys
Nickel-based alloys are divided into two groups (see GOST 5632-72): 1) alloys used primarily as heat-resistant, and 2) heat-resistant alloys that have the required minimum heat resistance (Table 7).
Table 6
Long-term strength and creep limits of iron-nickel based alloys *1
| Steel | Temperature, °C | Long-term strength limit, MPa for time, h | Creep limit*3, , MPa | ||||
| 100 | 500 | 1000 | 10 000*2 | 100 000*2 | |||
| KhN30VMT(EP437,VZh102) | 650 | 370 | — | 290 | 230 | 180 | 210 (1/104);14 (1/105) |
| 700 | 280 | — | 220 | 180 | 140 | ||
| 800 | 150-170 | — | 100-110 | 68 | — | ||
| ХН35ВТ(ЭИ612) | 600 | — | — | 320 | 270 | 230 | |
| 650 | — | — | 220-230 | 190-200 | 150-160 | 170 (1/104);130(1/105) | |
| 700 | — | — | 140 | 95 | 65 | 110 (1/104);80 (1/105) | |
| ХН35ВТУ(ЭИ787) | 600 | 650-680 | 550-580 | 520-550 | 420-450 | — | |
| 700 | 380-400 | 320-340 | 280-320 | 240-260 | — | ||
| 750 | 300-340 | 240-300 | 200-270 | 170-230 | — | 250 (0,2/100) | |
| 800 | 210-240 | 150-180 | 120-160 | — | — | 130 (0,2/100) | |
| ХН35В5Т | 650 | — | — | 280 | 200 | 160 | 180 (1/104);130 (1/105) |
| 700 | — | — | 200 | 150 | 120 | 120 (1/104);90 (1/105) | |
| 750 | 200 | — | 150 | 110 | 80 | 80 (1/104);60 (1/105) | |
| ХН38ВТ(ЭИ703) | 800 | 80-90 | — | 52 | — | — | 63 (5/100)*4 |
| 900 | 30-40 | — | — | — | — | 21 (5/100)*4 | |
| 1000 | — | — | — | — | — | 9 (5/100)*4 | |
| HN45Yu(EP747) | 1000 | 20 | — | — | — | — | |
| 1100 | 9 | — | 5 | — | — | ||
| 1200 | 5 | — | 2,5 | — | — | ||
*1 After optimal heat treatment.
*2 Extrapolated values.
*3 In brackets in the numerator - deformation in %, in the denominator - time in hours.
*4 Determined on conical samples.
Table 7
Limits of long-term strength and creep of nickel-based alloys*1
| Steel | Temperature, °C | Long-term strength limit, , MPa per time, h | Creep limits*3, , MPa | ||||
| 100 | 200 | 300 | 1000 | 10 000*2 | |||
| KhN65VMTYu(EI893) | 700 | > 600 | — | — | 400 | 300 | 300 (1/10 000) |
| 750 | 500 | — | — | 330 | 230 | 200(1/10 000) | |
| 800 | 300 | — | — | 200 | 140 | 120 (1/10 000) | |
| KHN70VMUT(EI765) | 600 | 780 | 750 | 740 | 650 | 530 | — |
| 700 | 450-500 | 420-470 | 400-450 | 310-350 | 220-240 | 200 (1/10 000) | |
| 800 | 220-250 | 210-230 | 190-220 | 140-160 | — | 80 (1/10 000) | |
| KhN70VMTYu(EI617) | 700 | 480-520 | — | 420 | 360 | — | 300 (0,2/100) |
| 800 | 280-300 | — | 210 | 180 | — | 170 (0,2/100) | |
| 850 | 180-200 | — | — | 100 | — | 170 (0,2/100) | |
| ХН80ТБУ(ЭИ407) | 650 | — | — | — | 400 | 300-260 | 350 (1/10 000) |
| 700 | — | — | — | 270 | 170-180 | 220 (1/10 000) | |
| ХН70МВТУБ(ЭИ598) | 700 | 480 | 420 | — | — | — | 180 (0,2/100) |
| 800 | 250 | 230 | — | — | — | — | |
| KhN67MVTYu(EP202) | 700 | 480-520 | — | 380-420 | 360-390 | 280-320 | 360 (1/1 000) |
| 800 | 280-300 | — | 230-250 | 180-200 | 120-150 | — | |
| 850 | 180-200 | — | 140-160 | 110-130 | 70-80 | — | |
| 900 | 120-140 | — | 90-100 | 70-80 | 40-45 | 60 (1/1 000) | |
| KhN75MBTYu(EI602) | 700 | 160-170 | 150 | — | — | — | — |
| 800 | 80 | 70 | — | — | — | 43 (5/100)*4 | |
| 900 | 29 | 22 | — | — | — | 14 (5/100)*4 | |
| ХН78Т(ЭИ435) | 700 | 105 | — | — | 32-35 | — | — |
| 800 | 45 | — | — | — | — | 18(5/100)*4 | |
| 900 | 15 | — | — | — | — | 7 (5/100)*4 | |
| KHN77TYUR(EI437B) | 600 | 680 | 660 | — | — | 450 | 720 (0,2/100) |
| 700 | 420 | 400 | — | 350 | 180 | 260 (0,2/100) | |
| 800 | 200 | — | — | 150 | — | 150 (0,2/100) | |
| ХН60У(ЭИ559А) | 800 | 60-80 | — | — | 40-50 | — | — |
| 900 | 35 | — | — | 20 | — | 24 (0,2/100) | |
| 1000 | 6 | — | — | — | — | 10 (0,2/100) | |
| ХН60ВТ(ЭИ868) | 800 | 110 | 95 | 87 | — | — | 83 (5/100)*4 |
| 900 | 52 | 43 | 40 | — | — | 34 (5/100)*4 | |
| ХН70У(ЭИ652) | 800 | 90-100 | — | 80 | — | — | — |
| 900 | 35-40 | — | — | — | — | 25 (5/100)*4 | |
| ХН75ВМУ(ЭИ827) | 850 | 270 (at least 50 hours); 250 (at least 65 hours) | |||||
*1 After optimal heat treatment.
*2 Extrapolated values.
*3 In brackets in the numerator - deformation in %, in the denominator - time in hours.
*4 Determined on conical samples.
The most commonly used alloys of the first group belong to the Ni-Cr-Ti-Al system. The presence of Ti and Al in these alloys in quantities exceeding their limiting solubility in solid solution at temperatures of 650-950 °C allows one to achieve a significant effect of dispersion hardening after quenching and tempering, due to the release of dispersed particles of the intermetallic phase such as Ni3(Ti, NiAl). This microstructure makes the alloy resistant to temperature effects at 700-800 °C and above.
The introduction of W and Mo (in total up to 10%), as well as Nb, into the dispersion-hardening alloys of this group additionally strengthens the solid solution, slows down the development of diffusion processes and increases the amount of dispersed strengthening phase. The amount of dispersed phase is also increased by increasing the total content of Ti and Al. All this leads to a significant increase in the heat resistance of alloys, which makes it possible to use them at temperatures up to 800-850 °C and high voltages.
Features of the composition of nickel heat-resistant alloys include the presence in them of small additives of surface-active elements (B, Ce, sometimes Ba and Mg), which contribute to the refining of the metal and the strengthening of grain boundaries, as well as a small content of impurities (S, P, Pb, etc.) .).
The heat treatment of these alloys consists of single or double heating to high temperatures (1080-1200 °C) with cooling, most often in air, and subsequent tempering at temperatures of 700-850 °C. For the greatest stabilization of the original structure, in relation to parts with a long service life, it is recommended to carry out multi-stage tempering at a gradually lowering temperature.
Heat-resistant nickel alloys are produced in the form of rolled products (round bars) and partly in the form of forgings of various configurations.
The main purpose of this group of high-alloy alloys is the manufacture of working blades and disks of gas turbines. Discs operate at higher stresses than blades (but at slightly lower temperatures), so the disc material must have high creep resistance (especially at the rim) and increased strength (at the hub).
The strength of nickel-based alloys remains high up to temperatures of 800-900 °C. Thus, at 800 °C, the tensile strength σв of the most alloyed alloys is 700-800 MPa, the 100-hour long-term strength is 250-300 MPa. At the same time, the plasticity characteristics δ and ψ are satisfactory at all test temperatures and decrease slightly in the temperature range of dispersion hardening (700-800 °C). The residual deformation of these alloys during long-term strength tests at 700-800 °C is about 3-10%.
In table Table 7 shows the heat resistance characteristics of nickel alloys.
For long service life, the best combination of long-term strength and ductility is found in the alloy KhN65VMTYu (EI893), which is widely used as a material for the blade apparatus of stationary gas turbines GT-6, GTN-9, GTK-10, GTK-16, GTT-12, GTA- 18, GTU-25, GTU-100. This alloy is the main blade material in stationary gas turbine construction. In addition, due to its exceptionally high relaxation resistance, this alloy is used for the manufacture of turbine fasteners.
Heat-resistant nickel alloys can be used to produce parts by casting (for example, precision investment casting).
The second group includes alloys of the grades KhN67MVTYu(EP202), KhN60Yu(EI559A), KhN70Yu(EI652), KhN78T(EI435), KhN60VT(EI868), KhN75MBTYu(EI602), used primarily as heat-resistant. These alloys, with the exception of the last two, are distinguished by a high Cr content (20-30%) and an almost homogeneous solid solution structure after the adopted heat treatment modes (heating to 1000-1200 °C with cooling in water or air). These alloys are produced in the form of cold-rolled or hot-rolled sheets mainly for parts of gas pipeline systems operating at moderate stresses under conditions of very high temperatures (up to 1100-1200 °C). In addition to sufficient manufacturability (rollability, stampability, weldability) and high resistance to gas corrosion (scale resistance), these parts must have good resistance to thermal fatigue (heat resistance). Nickel-based alloys meet all these requirements.
Heat-resistant sheet nickel alloys have increased ductility in cold and hot states, but the heat resistance is lower than that of alloys of the first group. Thus, long-term strength over 1000 hours is 40-60 MPa at 800 °C and 20-25 MPa at 900 °C (Table 7).
Classification
According to the accepted terminology, it is recommended to classify pearlitic steels according to the percentage of carbon they contain. Under the condition of equilibrium microstructure (meaning slow cooling, which excludes the formation of cementite Fe3C), steels are distinguished:
- Hypoeutectoid
- Eutectoid
- Hypereutectoid.
As already noted, hypoeutectoid steels contain no more than 0.6% carbon, and hypereutectoid steels contain more than 0.6...0.8%. In hypoeutectoid steels, the equilibrium microstructure at room temperature consists of ferrite and pearlite; this ferrite is called hypoeutectoid ferrite. Cooling from austenite (in the range from 875°C to 775°C) and further cooling to room temperature gives a microstructure consisting of hypoeutectoid ferrite and pearlite (isothermal transformation occurs at 727°C when austenite reaches eutectoid composition).
When the temperature drops below the eutectoid line (727°C), all austenite is converted to pearlite, with virtually no change in the hypoeutectoid ferrite structure produced during cooling. Proeutectoid ferrite is present as a continuous matrix phase surrounding isolated pearlite colonies. Ferrite is also present in pearlite and is known as eutectoid ferrite, which appears white in micrographs. The dark appearance of perlite is explained by the narrow composition of the microcomponents present in it.
Steel containing 0.8% C is known as eutectoid. The equilibrium microstructure obtained at room temperature is pearlite, which in turn is a mixture of ferrite and cementite. Ferrite is a very soft component and cementite is a very hard component of steel. This microstructure is obtained by equilibrium cooling from 800°C and has a lamellar structure.
The thick layers in the pearlite grain represent the ferrite phase, and the cementite phase appears as thin dark plates.
Pearlite has properties intermediate between soft plastic ferrite and hard brittle cementite. In hypereutectoid steel, the equilibrium microstructure at room temperature contains hypoeutectoid cementite and pearlite. The main difference from the hypoeutectoid structure is that there is a continuous network of cementite that separates each pearlite colony. As the carbon content increases, the thickness of the cementite network increases.
Receiving - granular perlite
| Cast steel structure. |
Normalization of steel
The production of granular pearlite is achieved by a special type of annealing, which is close in its regime to incomplete annealing. The steel is heated slightly above Ac, followed by cooling first to 700 C, then to 550 - 600 C and then in air.
To obtain granular pearlite (cementite in the form of grains), spheroidizing annealing is performed, which consists of heating the steel to a temperature slightly above the PS / C line (ACt point), long exposure (5 - 6 hours) and subsequent slow cooling. After such annealing, lamellar cementite turns into granular cementite.
To obtain granular pearlite (cementite in the form of grains), spheroidizing annealing is performed, which consists of heating the steel to a temperature slightly above the PSK line (point Ac), long exposure (5 - 6 hours) and subsequent slow cooling. After such annealing, lamellar cementite turns into granular cementite.
| Cast steel structure. |
Particularly important for obtaining granular perlite is precise adherence to the temperature regime, since with very slow cooling granular perlite is obtained with large grains, and often with individual perlite plates, and with rapid cooling, fine-grained (pointed) perlite is formed.
It is used to obtain granular pearlite and reduce hardness to improve the cutting machinability of steel with an O content of 0 6% and some grades of medium-carbon alloy steel. For example, in order to use high cutting speeds during rough and finish turning and preliminary milling of parts made of 35ХГС steel, spheroidizing annealing is used at 780, which results in a granular pearlite structure.
Partial annealing is used to improve machinability and obtain granular pearlite in the structure of hypereutectoid steels.
After forging, the rolls are annealed to prevent the formation of flakes and produce granular pearlite. Small rolls with a diameter of up to 210 mm are annealed simultaneously with an improvement in the forging condition.
| Structure of hardened and thermally cycled steel 01N18K10M5 - VD. |
In this state, steel cannot be processed by cutting; annealing is required to obtain granular pearlite. There are two annealing methods: isothermal spheroidizing annealing with long-term exposure at a constant temperature and pendulum annealing, when short-term exposures are performed alternately above and below temperature A. This structure of granular pearlite is achieved as a result of 3-fold accelerated heating in an oven to a temperature of 30 - 50 C above the Ac point, cooling in air to a temperature of 600 - 620 C and subsequent rapid cooling in water. Steel with such a structure is well processed by various cutting methods, and after final hardening and low tempering it has increased wear resistance.
| Scheme of isothermal and MICH processing FOR IC. |
Incomplete annealing of hypereutectoid steels is also called spheroidization, since this is the main method for producing granular pearlite. It was noted above that in order to obtain granular pearlite, heating should not exceed the critical point Ac by much, otherwise lamellar pearlite is obtained. Tool steels should have a granular pearlite structure, as this ensures good machinability with cutting tools and a low tendency to overheat during hardening.
To obtain high strength and ductility, pearlitic ductile iron castings are often annealed to produce granular pearlite. Annealing is used for castings with a high content of manganese and chromium (0 8 - 1 5 Mn, 0 15 - 0 2596 Cr), which eliminate the graphitization process during annealing. Annealing is carried out at a temperature of 720 - 740 C, followed by cooling in air.
The difference between group III is only a higher heating temperature for annealing and a longer isothermal exposure to obtain granular pearlite.
Production process
Optimization of the production of pearlitic steels is associated with the search for the best combinations of alloying elements: as we know, there should not be many of them, so the research is thorough.
In particular, to improve rolling, the maximum percentage of vanadium and silicon, elements that increase the performance characteristics of these steels, is regulated.
The results of mechanical tests show that such alloying additives have a beneficial effect on the mechanical properties of steels, especially those related to tensile strength. Silicon strengthens pearlite, mainly due to solid solution strengthening of the ferrite phase. Vanadium increases the strength of pearlite, mainly due to dispersion strengthening of pearlite ferrite. When added individually, these elements provide relatively greater strengthening at higher transformation temperatures. When added in combination (vanadium + silicon), the behavior is different and a significant increase in strength is achieved at all transformation temperatures studied (from 550°C to 650°C).
Dimensions of pearlite colonies
Annealing steel
An important characteristic of pearlite, which affects the properties of steels, is the size of the pearlite colony (Figure 3). A colony is a group of plates of cementite and ferrite that grew together, cooperatively, in austenite before colliding with other colonies
A decrease in the size of a pearlite colony is accompanied by an increase in the impact strength of steels and a decrease in their brittleness.
Increasing the brittle fracture strength of pearlite is achieved by spheroidizing cementite plates. This spheroidization can be achieved by deforming pearlite, followed by heating and holding at a temperature near the Ac1 point. Another method that provides relatively high strength and ductility to pearlite is to deform the pearlite during pearlite transformation. This leads to the formation of a polygonal structure and spheroidization of cementite.
Welding Features
Welding pearlitic steels, regardless of the method, usually does not encounter any difficulties. During welding, local melting occurs, re-solidification and subsequent cooling to room temperature.
The microstructure in the heat-affected zone includes a central melt region with austenite, which is obtained by local heating of pearlite. Provided that the weld is cooled slowly enough to room temperature, pearlite will form in the weld area. It may have a different grain size than the original material, but will have similar properties. At an increased cooling rate, the equilibrium phase transition does not occur, so martensite is formed from austenite in the melt zone. As a result, the weld becomes hard and brittle, which is undesirable for the mechanical connection of parts. To avoid this situation, the cooling rate is usually reduced or the weld is heat treated (tempered).
Thermal insulation boards made of perlite
Thermal insulation boards, which contain perlite sand and various binders (bitumen, lime, polymer compounds, cement, gypsum, clay, liquid glass), are manufactured by hydraulic pressing.
For normal positive and low negative temperatures, including areas of deep cold, perlite-bitumen products, such as slabs, are used.
The composition of perlite-bitumen slabs, which are used for thermal insulation of building structures and roofs of industrial buildings, includes perlite sand, bitumen, clay, asbestos, glue, sulfite-yeast mash (SYB) and water. Such perlite blocks can withstand temperature changes from -60 to +100 degrees Celsius and are divided into low-flammable (bitumen content is 9%) and low-flammable (bitumen content is 10÷15%).
The main advantages of insulating perlite slabs: low weight, high sound and heat insulation characteristics; resistance to rotting; resistance to deformation and mechanical stress.
Areas of application
Pearlitic steels in their initial state are well processed by cutting methods, therefore they are used as common structural materials, including those produced by stamping and welding.
If it is necessary to increase the strength properties, heat treatment is carried out, which consists of hardening followed by low tempering. It is carried out mainly in oil, which allows the austenitic transformation to be carried out most completely.
Currently, pearlitic steels are the most durable and at the same time ductile materials. However, it is not recommended to use them for the manufacture of products operating at high temperatures, since the heat resistance of pearlitic steels is low.
How to insulate a house using perlite
Perlite is used as insulation in the form of sand (bulk insulation); component in thermal insulation products and dry ready-made building mixtures.
Perlite sand as insulation for walls
Perlite sand for arranging thermal insulation of a house is an excellent material with which you can not only effectively insulate a home (heat loss is reduced by 50%), but also significantly lighten the structure of the building.
We begin installing thermal insulation from foamed perlite after part of the load-bearing wall (internal) and external brickwork (4-5 rows) have already been erected. We pour coarse expanded perlite sand (with a granule size of about 6 mm), previously dust-free, into the gap between these two walls and compact it thoroughly (the volume should decrease by 10%). We fill the sand manually or using a sandblasting machine. We repeat this operation several times until the walls are completely erected. By the way, in terms of heat-saving properties, a perlite layer about 3 cm thick corresponds to a 25 cm thick brick wall. When building panel houses, we pour sand between the sheathing sheets (internal and external).
If you are insulating an old house with voids in the walls, then backfilling with sand can be done in two ways:
- carefully pull out several bricks from the wall and pour perlite through the resulting hole;
- drill a hole in the wall (diameter 30÷40 mm) and through it, using a special installation, inject heat-insulating material.
Perlite sand is a universal non-combustible building material that has a number of advantages:
- excellent sound, noise and heat insulation properties (and can be used to insulate walls made of any material);
- environmental friendliness;
- lightness (by weight);
- resistance to temperature changes;
- durability.
Advice! You should not use perlite sand, which is a very moisture-intensive material, as insulation in places with high humidity.
The only disadvantage of sand is that it is very dusty: therefore, it is recommended to slightly moisten it before use.
Floor insulation with perlite
For thermal insulation of floors, we use expanded perlite, which is poured onto the cement-sand base of the floor and leveled according to building regulations. The height of the thermal insulation layer of sand is the desired thickness plus 20% additional volume for shrinkage.
Important! The recommended minimum thickness of the perlite layer is at least 1 cm
We embed uneven areas and pipelines in a layer of bulk material, and lay slabs and flooring on top. If there is no basement under the house, then in order for moisture to accumulate and be removed, we place drainage pipes and absorbent pads under the perlite.
Another effective way to insulate a concrete floor can be to lay a kind of “pie”: we install a perlite screed between two layers of concrete. First, prepare a perlite solution with the following components:
- cement – 1 mᶟ;
- perlite – 3 mᶟ (grade M75 or M100);
- sand – 2.2 mᶟ;
- water – 1.5 mᶟ;
- plasticizers – 3÷3.5 l.
Stir all the components of the mixture until water comes to the surface: this is a sure sign that the solution (perlite screed) is ready for use.
Advice! Since perlite is a very light material, it is recommended to carry out all work with this material indoors so that the wind does not in any way interfere with the work process.
After the perlite screed is applied to the concrete base, we leave it to harden. After 1 week we get an excellent thermal insulation layer for the floor that will last for many years. We lay a second layer of concrete on top of it.
Roof insulation
If you do not intend to equip a living space in the attic, then it will be quite enough to insulate only the attic floor with expanded perlite. Otherwise, we pour perlite between the beams of the roof slope into boxes that are specially made for this purpose; then compact the sand thoroughly. The work does not require specific skills or knowledge.
Also, for thermal insulation of sloping roofs, perlite is used, which is treated with bitumen in the factory. We add a solvent to this bituminized perlite and get an adhesive solution, with which you can create a durable thermal insulation layer.
Recommendations
- Raabe, D.; Choi, P. P.; Li, Y. J.; Kostka, A.; Sauvage, X.; Lecouturier, F.; Hono, K.; Kirchheim, R.; Pippan, R.; Embury, D. (2010), Extreme Strain Processed Metal Composites - Towards Ultimate Strength for Bulk Materials
,
35
, MISS Bulletin, p.982. - Li, Y.; Raabe, D.; Herbig, M. J.; Choi, P. P.; Go to page; Kostka, A.; Yarita, H.; Bochers, C.; Kirchheim, R. (2014), Segregation stabilizes bulk nanocrystalline steel with near theoretical strength.
,
113
, Physical Review Letters, pp. 106104, PMID 25238372. - Chen, Y. Z.; Csiszár, G.; Cizek, J.; Westerkamp, S.; Borchers, C.; Ungár, T.; Go to page; Liu, F.; Kirchheim, R. (2013, April 10). "Defects in the carbon-enriched ferrite of cold-drawn pearlitic steel wire." Metallurgical and Materials Operations
A.
44
(8):3882–3889. Doi:10.1007/s11661-013-1723-x. ISSN 1073-5623. S2CID 135839236. - Li, Y. J.; Choi, P. P.; Borchers, C.; Westerkamp, S.; Go to page; Raabe, D.; Kirchheim, R. (2011), “Deformation-induced decomposition mechanisms of cementite in pearlite at the atomic level,” Acta Materialia
,
59
(10): 3965, doi:10.1016/j.actamat.2011.03.022. - Alvarenga HD, Van de Putte T, Van Steenbergh N, Sietsma J, Therrien H (April 2009). "The influence of morphology and microstructure of carbides on the kinetics of surface decarburization of C-Mn steels." Metal Mater Trans
A.
46
: 123–133. Doi:10.1007/s11661-014-2600-у. S2CID 136871961. - https://www.engnetglobal.com/tips/glossary.aspx?word=Eutectoid+Steel
LOW-ALLOYED PEARLITIC STEEL (Khakimov A. N.)
8.1. Purpose of steels
Low-carbon, low-alloy steels of the pearlitic class are used in various structures instead of carbon steels, providing a reduction in metal consumption by 20-50%. They are widely used in the construction of pipelines, structures of gas and petrochemical plants, ships, bridges and other structures operated in the temperature range from -70 to +475°C, depending on the chemical composition and structural state provided by heat treatment.
8.2. Steel composition
One of the most effective means of improving the quality of low-carbon steels is their strengthening through alloying with elements such as Si, Mn, and increasing the dispersion of the structure through thermal or thermomechanical treatment.
The C content in low-alloy steels does not exceed 0.23%. Depending on the alloying elements, the total content of which in the steel composition does not exceed 5%, manganese, silicon-manganese, chromium-silicon-manganese and other steels are distinguished, presented in table. 8.1. In terms of S and P content, these steels can be classified as high-quality
CHEMICAL COMPOSITION AND MECHANICAL PROPERTIES OF LOW-ALLOY STEEL
Content of chemical elements,
Rolled thickness, mm
Fig. 8 I Determination of heating temperature depending on Sekv and thickness of welded rolled stock
To determine the heating temperature of steel in order to prevent the formation of cold cracks, depending on the content of chemical elements in it and the thickness of the rolled product, you can use the graphs shown in Fig. 8.1
[3]. The values of Seq, plotted along the abscissa axis, are determined as:
Seq = c + Mn/6 + Si/5 + Cr/6 +
+ Ni/12+Mo/4 + V/5 + Cu/7+ P/2.
Here the symbols indicate the percentage content of the corresponding chemical elements.
Their maximum content should not exceed 0.5% C;
1.6% MP; 1% Cg; 3.5% N1;
0.6% Mo; 1% Si. As can be seen, the required heating temperature increases with increasing degree of alloying of the steel and the thickness of the rolled product being welded.
Another methodological sequence, discussed in [3], makes it possible to differentiate the heating conditions for root and filling joints in accordance with the nomogram presented in Fig. 8.2.
The method for using the nomogram using the example of welding a root weld of steel 30 mm thick at a current value of 250 A, arc voltage 25 V, welding speed 25 cm/min is presented below:
We restore the perpendicular from point a, corresponding to / = 250 A, to the intersection with the straight line corresponding to the voltage of 25 V, and we obtain point b in quadrant I. Then we draw the horizontal line b - c to the intersection with the straight line corresponding to the welding speed of 25 cm/min, c quadrant //. After this, we lower the perpendicular from point c until it intersects with the curve corresponding to the rolled thickness of 30 mm, and we obtain point g; then draw the horizontal line d - d until it intersects with straight line 1, corresponding to the conditions for welding the root weld in quadrant IV, restore the perpendicular from point to point e and determine the heating temperature corresponding to 150 ° C
Rice. 8.2. Nomogram for determining welding modes and preheating of steels containing SEQ
Special steels: types, alloying impurities
To give steel special qualities, special impurities are used, which are called alloying elements. They are introduced into the alloy composition during the smelting process when certain conditions are created. Nickel, chromium, titanium, cobalt, molybdenum, aluminum and others are used as such substances. As a result, chromium-nickel, manganese, cobalt, titanium steels and the like are obtained. For carbon steels, manganese and silicon are mainly used, since it is these components in the required proportions that impart the desired properties to such alloys.
WELDING AND WELDED MATERIALS
POROUS MATERIALS ON A METAL BASE (Tretyakov A. F.)
39.1. Classification of porous materials Porous materials (PM) on a metal basis are used as filter elements, mixers, gas lenses, noise suppressors, etc. PM are classified according to their purpose, chemical composition and type of structure-forming ...
COMPOSITE MATERIALS WITH A METAL MATRIX (Chernyshova T. A.)
38.1. Classification Composite materials are materials reinforced with fillers arranged in a certain way in the matrix. Fillers are most often substances with high energy of interatomic bonds, high strength and high modulus, but in combination ...