Feature of ZV Pu-Zinc system:
1. Application up to 98% humidity.
2. Drying time for ZV zinc paints to “touch” is 30 minutes.
3. Instant resistance to fresh and sea water.
4. Long service life of the zinc anti-corrosion “working” film.
5. Can be applied to an unprepared surface.
6. Extreme adhesive performance of ZV zinc paint.
7. Provides cathodic protection of the metal.
8. Our zinc paints are oil and grease resistant.
9. It is recommended to apply ZV zinc compounds at temperatures from -5°C to +50°C.
10. ZV is compatible with other polyurethane and epoxy compounds.
Purpose
Zinc-containing paint is most often used for painting ferrous metals that are used in difficult weather conditions. These compositions cover:
- building structures;
- bridge structures;
- ships;
- metal tanks;
- metal pipes;
- gas pipelines;
- agricultural machinery;
- metal pillars;
- tunnels;
- port facilities.
It is not prohibited to use paint with zinc in everyday life. It can be used to paint water pipes or heating radiators. If the paint functions as a primer, it is applied in 1-2 layers, and if as a coating - in 2-3.
Types of body galvanization
Specialists use several technological options for galvanizing of varying complexity of processing, cost and durability:
- Hot (thermal). The operation is not available for garage repairs. The entire car body or only a certain part is lowered into a tank with a zinc alloy; during rental, a protective layer is applied on top. The most effective method of protection.
- Cold galvanized. A budget and easy to implement option, but the least effective. It takes place in two stages: applying a chemical layer; coating with a composition that contains tiny particles of zinc.
- Galvanic. The most common option. You can carry out the process yourself. A car part is lowered into a container with a special composition containing zinc. Under the influence of an electric discharge, the protective layer is fixed. An example is galvanized body washers.
- Zinc metal. The sheet metal is treated with a coat of zinc-based epoxy paint with a secondary coating of rust inhibitor. The main advantage of this option is that the anti-corrosion properties are not lost during mechanical processing.
Types of body galvanization depend on the degree of processing of the machine:
- full – the whole machine is galvanized;
- partial - only some parts were galvanized.
How much does the procedure cost?
According to the latest data, complete hot galvanization in a service center costs from 25,000 rubles. The difficulty is that only 5% of service stations carry out complex galvanizing with complete removal of the body, cleaning, priming and subsequent application of paintwork.
Partial galvanizing of small parts or body parts will cost from 5,000 rubles. The cost of the kit for use in the garage and independent work is 800 rubles. and more.
Do-it-yourself galvanizing of metal protects your car from corrosion by 98%. When self-galvanizing component parts, this is the only reliable and longest-lasting method of protecting the part, which can be used at any time. It is enough to carry out a full inspection twice a year and update the partial cold galvanization of the body and galvanization for removable parts.
If noticeable pockets of corrosion have formed on the body of your truck or car, but they are still not through, you need to take immediate action. As practice shows, if you do not stop body corrosion in time, the cost of subsequent repairs to your car will be many times higher. For this reason, you can’t hesitate!
In recent years, many motorists have noticed: even in large densely populated cities, road services mercilessly use salt. As a result, the bodies rot to holes in literally two to three years. To resist the rusting process and the aggressive effects of road reagents, there is a very simple but effective way - do-it-yourself galvanizing of the body.
Galvanizing a car is carried out in order to protect the body once and for all from the formation of annoying “saffron caps”, as well as to prevent the further spread of corrosion throughout the body.
The zinc “crust” on the surface of the metal creates a kind of barrier that protects the steel from negative factors and the destructive effects of an aggressive environment. The zinc-based coating effectively resists the effects of salts, chemicals and moisture.
Please note that you can galvanize both a part of the car body (fender, trunk, hood, etc.) and any individual part. And this will require direct hands, certain knowledge, a specific set of materials and tools, as well as the desire to give your car additional strength.
The “home” method of galvanizing metal allows you to prevent the formation of corrosion on the car body and thereby reduce service costs. Liquid galvanizing can also be used for various metal products to further protect them from rust.
It must be said right away that this method is very simple and does not require large financial costs. In fact, this is the well-known Zinkor car, but with your own hands. This miracle remedy will be written in detail below, as the article progresses.
Preparatory work
This method involves the use of phosphoric acid with zinc dissolved in it, and will also require zinc (salt) batteries. You can use both small finger batteries and large batteries - in this case it all depends on the amount of work being done.
If you need to galvanize a large surface area on your car, then it is better to take large zinc batteries. First, you need to “print” them all and remove all the excess braiding.
If you wish, you can also use only the casing of a salt battery, after removing the graphite core and soot, but in principle you can leave all the “internals” in place.
On the body of the “bare” galvanized battery, on one side you need to secure a cotton pad with an elastic band, and on the other side (also using a regular rubber band) - the power wire. You can use a car battery or a suitable power supply as a power source.
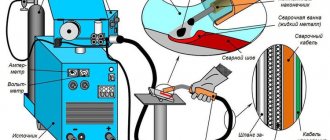
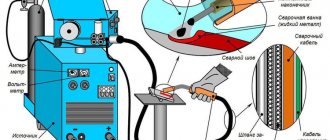
How does galvanizing of a surface occur?
If you don't know how to galvanize correctly, read the short instructions first.
The “minus” from the car battery must be connected directly to the part of the body (or part) that you are going to galvanize with the battery - that is, using the battery.
We connect the wire that goes to the zinc body of the battery to the positive terminal of the battery. Please note that the negative terminal should never be disconnected, because the desired effect will not be obtained.
Before starting galvanizing, it is advisable to clean the surface to be treated from traces of rust, if any. You need to take orthophosphoric acid with zinc dissolved in it into a syringe and soak a cotton pad that is placed on the body of the salt battery. After this, you just need to move the “nozzle” over the entire area of the surface being treated.
The most important thing is not to stop. If you keep the battery in one place for a long time, then burns appear, and if you move continuously over the surface, you get a neat and even layer of zinc. You will see the result almost from the first seconds.
Although some people call this method of galvanizing a car “artisanal,” it is truly a proven, simplest and, most importantly, effective way to combat corrosion. And, as an option, you can use a ready-made camping kit - a car kit for galvanizing Zinkor auto.
Using this product, you can quickly localize particularly advanced areas of rust, as well as remove all traces of corrosion on the car body. Galvanic galvanization allows you to reliably protect the metal surface from the reappearance of “saffron caps”.
Method of galvanizing metal using “white powder”
Typically, for galvanizing small areas of metal surfaces, phosphoric acid and a galvanized battery casing are most often used. However, for better processing, it is better to use soldering acid instead of phosphoric acid. It is hydrochloric acid in which zinc is dissolved. It is believed that the galvanization of the body in this case will be deeper and more durable.
You can purchase soldering acid at almost any radio electronics store. But it’s not entirely convenient that soldering acid for soldering at home is sold only in small bottles.
Therefore, if you need large volumes of soldering acid for galvanizing, it can be made at home from “white powder” - zinc chloride, which is sold by weight.
Main stages of work
Pour zinc chloride into a suitable container, then add distilled water and mix until a clear liquid consistency is formed (note that you need to wear rubber gloves when working with chemicals). From 1 kg of zinc chloride, approximately 3.5 liters of finished soldering acid are obtained.
The result is a galvanic bath, in which, if necessary, pieces of sheet steel and entire metal parts can be galvanized.
For further work, you will need a galvanized battery case and an iron bolt, at the ends of which cotton fabric must be secured with rubber bands.
The part that needs to be galvanized must first be thoroughly cleaned of rust with a grinder, using a cleaning disc with a metal brush. Before galvanizing, the metal must be “activated” - the oxide film must be removed from the surface using electricity.
Types of zinc paint
Protective agents against metal corrosion containing zinc are presented on the market in two versions, which differ in the binding components:
- with organic binders;
- with inorganic binders.
Zinc coatings with binders of organic origin are:
- epoxy;
- alkyd;
- urethane;
- chlorinated rubber.
Compositions with inorganic substances are presented in the form of silicate materials that contain zinc.
General information about zinc-containing paints
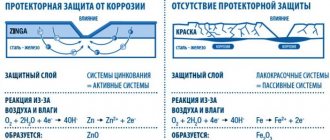
Paints and varnishes containing a high percentage of zinc (80-95% and above) provide metal objects with long-term protection against rust. Zinc-containing, or rather zinc-filled, paints are used for painting or priming metal. They are applied to the iron base with a brush, roller, or spray gun. Painting metal with zinc-containing paints is called cold galvanizing. This method is an alternative to hot-dip galvanizing.
After applying zinc-containing paintwork materials to the base, a corrosion-resistant film is formed. Zinc in the paint coating prevents moisture from destroying the iron. Zinc powder together with resins creates an anti-corrosion barrier on the painted surface.
True, after applying zinc-containing paint, the fresh coating still contains micropores that allow moisture to pass through to the iron (promoting the formation of rust). However, soon as a result of the oxidation reaction, zinc oxides and zinc bicarbonates are formed. A zinc film is formed, filling the smallest pores and “healing” defects on the metal surface. During another, electrochemical reaction, zinc carbonate is formed. This is also a water-resistant film.
The zinc coating has the ability to self-repair if its integrity is compromised during operation. Penetration of moisture provokes an oxidative and electrochemical reaction. As a result, a new film and a new anti-corrosion barrier are formed.
Not every zinc-containing (zinc-rich) paint can be used for cold galvanizing. It is advisable to buy not paintwork materials with zinc, but zinc (fine powder 3-5 microns (88%) or highly dispersed powder 12-15 microns (94%)) with the addition of resins and solvents. Such compositions are often called zinc primer. Another name for them is liquid zinc. Simple zinc-containing paints with a low percentage of zinc powder do not provide a long-term anti-corrosion effect.
Cold galvanizing method
It is not difficult to paint with paint containing zinc on your own, the main thing is to adhere to consistency in the process. Painting is no different from painting with conventional paints and consists of the following steps:
1. It is necessary to clean the surface of old finish, rust and other dirt. You can use regular sandpaper or a wire brush to complete this task. The coating must be clean after cleaning.
2. Prepare the coloring composition by stirring it thoroughly until a homogeneous structure is formed. If you need to thin the paint, you need to use a solvent, the type of which is indicated on its packaging.
3. The material is applied in a thin layer using a painting tool (brush, roller, spray gun). There is also paint on sale in aerosol form, which is applied evenly without forming smudges or streaks.
4. Re-application of the coloring composition is carried out after the previous layer has completely dried.
5. The final finishing process can be coating with decorative paint.
As a result of painting, the surface becomes 95% covered with zinc.
On video: preparing metal for galvanizing.
Areas of application
The cold galvanizing method is used for painting:
- metal objects used outdoors;
- metal bridges, hydraulic structures, power line supports, road fences;
- radiators, batteries;
- pipes, rolled metal, containers, tanks;
- vehicle bodies, ship hulls;
- building metal structures;
- gates, fences, doors, metal elements;
- to restore a previously galvanized surface;
- water, gas pipes and heating mains.
How to choose a composition?
Before deciding to purchase a specific composition for cold galvanizing, it is necessary to study its content and product quality. There is practically no possibility to choose by color; it is mostly a gray-matte shade. The material consumption does not differ much from the type of paint and the type of its application; on average, it amounts to 300 grams per square meter.
To choose a high-quality composition for galvanizing, you need to pay attention to the following:
- product cost;
- shelf life of the product;
- time for complete drying of 1 layer;
- amount of zinc in the composition;
- conditions under which the surface is processed;
- service life of the protective coating.
Cold galvanizing is a popular method among specialists for protecting metal products, which can be done at home with your own hands. With proper surface preparation, high-quality finishing materials and following the advice of the experts, metal galvanization will take place without problems.
Cold galvanizing technology with Galvanol composition (1 video)
Zinc-based paint of different brands (20 photos)
Varieties
Zinc-containing coatings, in addition to zinc, contain resins: organic (epoxy, alkyd, chlorinated rubber, urethane) or inorganic (silicate). Paints and varnishes for cold galvanizing can be one-component or two-component. Compositions consisting of two semi-finished products are combined with each other and mixed before use.
Epoxy
Epoxy resin-based coatings are considered the most durable. Zinc-containing compounds containing at least 85 percent zinc powder are used to protect objects in the oil, gas, energy industries, and waterfowl from corrosion.
form an anti-corrosion coating of increased strength;
have a long service life.
toxic composition;
high consumption.
Alkyd
The most common coatings containing zinc. Available in the form of a spray or liquid paint in cans. Used to protect metal elements and structures from rust.
the coating is resistant to adverse weather conditions and moisture;
Coatings with zinc can be applied over a painted base.
toxic composition;
relatively high consumption;
Urethane
Zinc-rich urethane or polyurethane coatings are used to protect metal objects from rust. May contain up to 96 percent zinc. Suitable for cold galvanizing.
creates a durable anti-corrosion film;
has a long service life.
toxic composition;
high consumption.
Chlorinated rubber
This is a zinc-containing primer based on chlorinated rubber. Creates a coating that is resistant to moisture, acids, and petroleum products.
forms a durable anti-corrosion film;
protects metal objects from adverse weather conditions.
the coating is not resistant to organic solvents;
the paintwork itself has a toxic composition.
Silicate
Typically these are two-component heat-resistant compounds. They are used to protect metal objects that heat up during operation from rust.
durable anti-corrosion film;
durability of operation regardless of the thickness of the coating;
toxic composition;
surface preparation for painting is necessary.