Fechral and nichrome: comparison of alloys
The alloys fechral and nichrome are quite similar, but according to many craftsmen, preference should be given to nichrome. Fechral and nichrome belong to the group of precision alloys, that is, they have precisely defined characteristics. Although both of the above alloys have significant value for industry due to their inherent qualities, there are differences that affect the “fulfillment” of the tasks assigned to them.
So, for example, fechral is considered more fragile than nichrome, since it does not contain nickel (Ni), which tends to increase the strength and survivability of the alloy in watches.
Magnetic alloys
These alloys are widely used in electrical engineering. They are used to make permanent magnets, transformer cores, electrical measuring instruments, and electromagnets. People have long known that iron has magnetic properties and as a result it has many uses.
Much later it was discovered that the same property is inherent in nickel and some other metals. Products made from a magnetic alloy of iron and nickel also have the ability to retain their own magnetic field when the external one is no longer present. Moreover, this personal field is again capable of influencing other magnetic bodies.
Fechral - an alloy of iron, chromium and aluminum
Fechral is a precision alloy based on iron (Fe). It also contains chromium (Cr) and aluminum (Al). In addition, some other elements are present in a small percentage.
This alloy can have several modified compositions and, accordingly, grades. The most common are fechral X23YU5T, X27YU5T, and X15YU5.
As mentioned earlier, the most significant component is iron (Fe). It is about 70% in Kh23Iu5T and 66% in Kh27Iu5T.
The price of fechral is lower than nichrome, precisely due to the high iron content. The element (Fe) degrades some characteristics of the alloy.
The next component is chromium (Cr). Its content is 23-25% and 27-28%, respectively.
Aluminum (Al) in X23Iu5T and X27Iu5T is contained in almost equal quantities - from 5% to 6%.
The X15Yu5 grade of fechral is characterized by a high iron (Fe) content, close to 80%, and a reduced chromium (Cr) content - from 13% to 15%.
Thanks to this, the alloy gains strength, but not as much as that of nichrome. The percentage of aluminum does not differ much from other brands, it is no more than 6%, no less than 4%.
All grades of fechral also contain nickel (Ni), titanium (Ti), silicon (Si), manganese (Mn) and carbon (C). Their share is up to 1%. It also contains calcium (Ca), cerium (Ce), phosphorus (P) and sulfur (S) in very small quantities.
Kovar alloy
The mixture consists of metals with excellent mechanical properties. They are easy to process and can be easily rolled, drawn, forged and stamped. An alloy of cobalt, nickel and iron is otherwise called kovar. A well-chosen combination of chemical elements provides the material with excellent characteristics. This alloy has good thermal conductivity, a high coefficient of electrical resistivity and linear expansion rates close to zero over a wide temperature range. The only drawback is low corrosion resistance in damp environments, so protective coatings made of silver are often used. Kovar is widely used in industry for the production of:
- pipes, tapes and wires;
- capacitors;
- equipment housings in instrument making;
- parts in radio electronics;
- housings in the electrovacuum industry.
The content of expensive cobalt and nickel in the alloy increases the cost of the material, but good characteristics and long-term operation cover the initial investment.
Pros and cons of fechral
The presented alloy, the main components of which are iron, chromium and aluminum, has its advantages and disadvantages.
Advantages:
- low electrical conductivity;
- cheaper than nichrome;
- has a high operating temperature (up to 1400 °C);
- good heat resistance.
Flaws:
- too fragile and short-lived;
- highly magnetic (caused by high iron content);
- susceptible to oxidation;
- Winding is possible only in a heated state.
Introduction.
The issue of corrosion of aluminum with a nickel-phosphorus chemical coating is of great importance in technology. Aluminum itself is difficult to coat and there is always a risk of the coating peeling off, even over time. Corrosion under the coating can speed up this process significantly. The corrosion mechanism of coated aluminum is complex and multifaceted; to understand it, it is necessary to use electrochemical studies, electron microscopy and X-ray phase analysis. Electrochemical tests were carried out under simulated cathode and anodic conditions simulating PEMFC (polymer electrolyte membrane fuel cells) fuel cell systems.
Nichrome - the power of nickel
Nichrome is also included in the group of precision alloys. Mainly composed of nickel (Ni) and also chromium (Cr).
The approximate percentage of each element in this alloy: nickel (Ni) 55-80%, chromium (Cr) 15-23%, manganese (Mn) no more than 2%. Also, a very small percentage is occupied by silicon (Si), titanium (Ti), aluminum (Al) and carbon (C), and even smaller amounts contain phosphorus (P) and sulfur (S).
Nichrome also has several grades, depending on changes in composition. Thus, nichrome brands such as X20N80 or X15N60 are widely used.
“X” means chromium, the numbers immediately after this letter are the percentage of chromium, respectively. So exactly “N” is the percentage of nickel. There are also brands with zirconium (Zr) additives - nichrome X20N60-N and X15N60-N.
By the way, the name of the brand can often hide the method of creating the alloy. For example, the names Nichrome X20N80-VI and Nichrome X15N60-VI imply that VI is a vacuum-induction production of the alloy.
Use of nickel in pure form
To protect metals from corrosion
For this purpose, coatings are used that are applied by electroplating or cladding. The first method is used for aluminum, cast iron, magnesium and zinc, the second - for unalloyed steels and iron.
For the production of metal products that have permanent shapes and high corrosion resistance
Nickel in its pure form is more expensive than iron and steel, so it is used in cases where it is impossible to get by with another metal with a nickel coating. Nickel is used to make crucibles and boilers, tanks for transporting and melting alkalis, storing reagents, food products, etc. Condensates are made in nickel pipes. Tools made from this metal are resistant to interaction with aggressive elements, so they are practically indispensable in chemical laboratories and medical centers. Various nickel devices are used for television, radar and nuclear technology.
As catalysts and filters in the chemical industry
Nickel has the same catalytic properties as palladium, but costs significantly less, so it is widely used in powder form in the hydrogenation reactions of alcohols, unsaturated and aromatic hydrocarbons, and cyclic aldehydes.
Pure nickel powder is also suitable for creating porous filters, which are used to filter various products: fuels, gases, etc.
For mechanical neutron beam choppers.
The properties of nickel make it possible to obtain high-energy neutron pulses, as a result of which plates made of this metal are used in nuclear physics.
Nickel is also used in the manufacture of electrodes in alkaline batteries.
Fechral and nichrome: physical properties
The presented alloys have specific physical properties. The density of fechral is 7.2 g/cm3, nichrome - 8.4 g/cm3.
The resistivity is 1.2-1.3 μOhm m and 1.0-1.1 μOhm m, respectively.
Fechral is ferromagnetic, while nichrome is non-magnetic.
The specific heat capacity of fechral is 0.48 °C, and that of nichrome is 0.44 - 0.46 °C. Hardness is 200-250 units and 140-150 units, respectively.
The maximum melting point of fechral is 1500 °C, nichrome is 1400 °C.
Oxidation resistance
Due to the nickel composition, nichrome practically does not undergo oxidation. During the heating period, a thin but protective film (of chromium oxide) is formed on the surface of the nichrome part, which quite significantly increases the durability of the alloy in more severe conditions.
In turn, fechral will be subject to oxidation much more strongly, since the main component of the alloy is iron. During oxidation, a more massive, high-density oxide film is formed on the surface of the fechral alloy. This film appears much faster on fechral than on nichrome.
Alni alloys
Alni is the group name for iron-nickel-aluminum magnetic alloys. As the concentration of aluminum and nickel increases within certain limits, the residual induction decreases and the coercive force increases. The most commonly used alloys are those containing 11 to 18% aluminum and 20–34% nickel. The main properties of such alloys are electrical conductivity, thermal conductivity and ductility. All of them are characterized by good welding.
To use alloys in the manufacture of magnets, they are alloyed with cobalt and copper. In this case, the material becomes hard and brittle and has a coarse-grained structure. Alni alloys are used as a structural material for parts of gas turbine and jet engines operating under high temperatures of more than 1000 degrees Celsius for a long time, preserving the metal without damage.
Hardness and ductility under normal conditions
Higher ductility is characteristic of nichrome. This percentage indicator for the alloy is approximately 20, but this may also depend on the marking.
Fechral has a ductility that is approximately 16%, but this is for the X15Yu5 brand. But the alloy marked X23Yu5T has even lower ductility - 10%.
But fechral is more durable because it contains a large amount of chromium. This does not allow it to be as flexible as nichrome.
Fechral is more brittle because it contains a high percentage of chromium.
Winding of fechral is available only at high temperatures (from 300 °C), and nichrome can be wound into a spiral at ordinary room temperature.
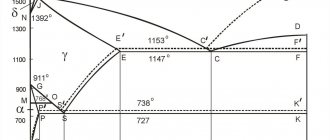
A little history
Invar is an alloy of iron and nickel, which contains 36% alloying additive. It was first discovered in France in 1896 by physicist Charles Guillaume. At this time, he was working on the search for inexpensive metal for standards of mass and length measures, which were made from a very expensive platinum-iridium alloy. Thanks to this discovery, the scientist received the Nobel Prize in physics in 1920.
The word "invar" in Latin means unchanging. This means that the coefficient of thermal expansion of an iron-nickel alloy remains constant over a wide temperature range - from -80 to 100 degrees Celsius. This alloy has several other names: nilvar, vakodyl, nilo-alloy, radiometal. Invar is a trademark of Imphy Alloys Inc., which is owned by the steel company Arcelor Mittal.
Scope of application of fechral and nichrome
Nichrome is considered a very durable alloy. This played a big role in the manufacture of different diameters of spirals and wires. Rods, nichrome threads, tapes, sheets, and strips are also made from nichrome.
Due to their high heat resistance, it is permissible to use nichrome alloys in the production of heating devices.
Nichrome retains its key physical properties even with strong temperature fluctuations, which makes it possible to use the alloy quite widely.
Tubular heating elements are made from nichrome and fechral. These alloys can be worked at a maximum temperature of 1400 °C and 1500 °C, respectively. Therefore, these alloys can be used in the production of rheostat elements and wire resistors.
Fechral and nichrome are used in similar areas, despite their different composition and distinctive characteristics.
About the properties of iron
Pure iron is silver-gray in color and has ductility and malleability. Native ingots found in nature have a pronounced metallic luster and significant hardness. The electrical conductivity of the material is high; it easily transmits current with the help of free electrons. The metal has average refractoriness, softens at a temperature of +1539 degrees Celsius and loses its ferromagnetic properties. This is a chemically active element. At normal temperatures it easily reacts, and when heated, these properties increase. In air it becomes covered with a film of oxide, which interferes with the continuation of the reaction. When exposed to a humid environment, rust appears, which no longer prevents corrosion. But despite this, iron and its alloys are widely used.