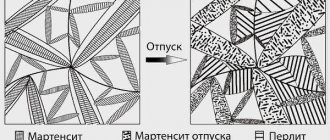
Cyanidation of steel is one of the ways to improve the physical and chemical properties of the metal. The use of the method is necessary when it is necessary to increase the strength, hardness, corrosion resistance, wear resistance of the surface layer of steel, and make it more resistant to natural aging.
Nitrocarburization strengthens steel by exposing it to carbon and nitrogen, or rather, introducing these molecules into the crystal lattice of the surface layer. This entire process occurs under the influence of high temperatures in an environment of sodium cyanide salts, the oxidation of which leads to the release of carbon and nitrogen.
How deeply the cementitious substances penetrate into the metal structure and what degree of concentration is formed depends on the selected temperature of the operation and the time interval of exposure. Nitrocarburizing and cyanidation of steel are operations that pursue the same goal, but take place in different environments.
Cyanidation, nitrocarburization
This is a technology for saturating steel with nitrogen and carbon.
In this way, steels with a carbon amount of 0.3 - 0.4% are processed. The ratio between carbon and nitrogen is determined by temperature. As it grows, the share of carbon increases. If oversaturated with both elements, the layer becomes brittle.
The layer size is affected by exposure time and temperature.
Cyanidation is carried out in liquid and gaseous media. The first method is also called nitrocarburization. In addition, according to the temperature regime, both types are divided into high and low temperature.
In the liquid method, salts with sodium cyanide are used. The main disadvantage is their toxicity. The high-temperature version differs from carburization in speed, greater wear resistance and hardness, and less deformation of the material. Nitrocarburization is cheaper and safer.
Nitrocarburization of steel
The final mechanical treatment is first carried out, and the fragments that are not subject to cyanidation are covered with a layer of copper 18 - 25 microns thick.
Advantages, disadvantages
When choosing a processing method, it is necessary to take into account the thickness of the products, since thin objects subjected to cyanidation may be more fragile than parts processed using conventional carburizing technology. This is a disadvantage of the technology under consideration. In addition, as a result of such processing, the properties of not the entire material change, but only its surface layer up to 1.6 mm thick. Finally, during cyanidation, constant monitoring of the degree of carburization and nitriding of the working environment is necessary.
The main positive feature of the processing technology under consideration is the relatively low temperature regime. Firstly, it simplifies implementation by not requiring the product to be cooled upon completion. Secondly, it increases the reliability of equipment, reducing its wear. Thirdly, it does not cause deformation of the processed objects. In addition, the material subjected to cyanidation contains retained austenite, which helps to improve many parameters of steel, namely, the impact strength of surfaces, wear resistance, bending strength, and ductility increase. In addition, cyanidation increases the hardness (up to 58 - 62 HRC) and contact endurance of the material. Also, parts subjected to gas cyanidation are characterized by improved hardenability due to increased stability of the austenitic structure of the steel. For example, low-alloy steel after such treatment can be quenched in oil.
Cyanidation of high speed steel
During the cyanidation process, the surface of high-speed steel is saturated with carbon and nitrogen to a depth of 0.02-0.03 mm and acquires very high hardness (up to 1110-1200 HV). Therefore, a cyanogenized tool has less adhesion of the treated metal to the surface, which makes it lighter for the chips skip.
Liquid cyanide is usually carried out in a bath of 30-50% sodium (or potassium) cyanide and molten salt containing 50-70% soda or other neutral salts. The exposure time of cyanide in a liquid medium must be sufficient to warm the steel to the bath temperature and completely dissolve the cyanide salt crust that forms on the surface of the product when the product is immersed in molten cyanide salt.
Typically, cyanosis in a liquid bath lasts no more than 10-15 minutes for small instruments and 20-30 minutes for large ones. Cyanide gas is carried out in a closed furnace, into the working space of which a mixed gas is supplied, consisting of 20-30% ammonia and 70-80% generator, gas with pyrolysis or other carbon.
For cyanide gas, the exposure time is much longer than for liquid, ranging from 1 to 2 hours, depending on tool size, load size, etc. Many low temperature gas cyanide factories use several modified pyrolysis mine cements in high speed steel tools furnaces where carburizing gas is obtained by pyrolysis of liquid hydrocarbons, which are fed by dropping into a retort where high-speed steel is located, and ammonia is fed through special tubes from the bottom of the retort and mixed with a gas fan.
The process of low-temperature cyanidation of gas with triethanolamine is very promising. Triethanolamine is a thick, viscous, yellow liquid with the chemical formula (C2H4OH) 3N, its composition: 48.5% C, 9.5% N2, 10% H2 and 32% O2, specific gravity 1.1, boiling point 277°. When triethanolamine falls into a preheated furnace, carbon-nitrogen gas can be produced directly in the working retort without ammonia. The presence of oxygen in triethanolamine prevents graphitization during cyanidation. This happens because the carbon released in this way gives oxygen to carbon monoxide, which also participates in the carbonization of steel. This process is inefficient, very inconvenient for production, and is also susceptible to damage during heat treatment.
Compared to cyanidation of liquids and gases, the result is worse. Solid cyanide can be carried out, for example, in the form of a powder consisting of 30-40% yellow blood salt and 60-70% carbon carburant. The instrument is packaged in an iron box and heated in an oven at 560-570° for 1-2 hours. And then he is released into the air. High-speed steel cyanide is carried out at a temperature 15-20° lower than the tool's tempering temperature, so as not to reduce the hardness of the basic metal structure during cyanidation. If the cyanide plating mode is not correct, for example, the exposure time in the cyanide environment is too long, carbide and nitride crusts may form on the surface of the tool.
This crust is very fragile, and the blades of a tool of this structure become stained during operation. It is very convenient to expose the tool to high temperature cyanide before quenching to protect the steel from decarburization and extend the life of the tool during operation. Heat for 15-20 minutes. At 820-850° in cyanide a layer of cyanide bath can be obtained. It is well preserved even with further hardening and tempering of the tool.
| Defects during heat treatment of high-speed steel | Cold processing of hardened high-speed steel |
| Alloys of the Fe - Cu system | Isothermal hardening of high-speed steel |
Pereosnastka.ru
Nitriding and cyanidation
TO
category:
Metals
Nitriding and cyanidation
Nitriding is the process of saturating the surface layers of a product with nitrogen. It imparts high hardness, increases wear resistance and fatigue strength of parts.
Before nitriding, the alloy steel product, typically containing chromium, aluminum and molybdenum, is hardened with a high sorbitol tempering and fully machined. Then it is placed in an oven, which is a hermetically sealed muffle with electrical heating, where it is processed in an atmosphere of partially dissociated ammonia.
At the end of the nitriding process, the furnace is turned off and the part is cooled under a current of ammonia to a temperature of about 200°. After nitriding, the part is not subjected to additional heat treatment and, after light grinding, is sent for assembly.
It can be seen from this that nitrided parts, unlike cemented parts, first undergo heat treatment and then undergo nitriding.
Due to this sequence of operations, nitrided products are free from the disadvantages inherent in cemented parts undergoing heat treatment after carburization.
This is the most important advantage of nitriding compared to carburization. Due to the low nitriding temperature, the steel product retains its sorbitol structure.
Diffusing into the metal, nitrogen not only forms nitrides of chromium, aluminum and molybdenum, but also dissolves in sorbitol ferrite.
The limiting solubility of nitrogen in iron occurs at a temperature of 590° and is 0.42%, which is many times higher than the solubility of carbon.
Nitrogenous ferrite is included in the sorbitol of the surface layer containing high-hardness nitrides. This structure imparts the specified high hardness to the surface layer.
The nitriding rate at a temperature of 525° is approximately 10 times less than the carburization rate at 925° and is 0.01 mm per hour. In nitriding practice, layers with a thickness of 0.25 to 0.7 mm are usually obtained. Nitriding to a depth of 0.7 mm lasts 70-90 hours.
The long duration of nitriding is its major disadvantage. However, high hardness and fatigue strength, as well as the absence of warping, are the reasons for using this method to strengthen a number of critical parts.
Steels for nitriding
Along with steel 38ХМУА, steels with nickel, tungsten and vanadium are also currently used.
The main ones are steel grades 18KhNMVA and 38KhNMFA, containing on average, respectively, carbon 0.18 and 0.38%, chromium 1.5%, nickel 4 and 1.5%, molybdenum 0.2 and 0.3%.
In addition, 18KhNMVA steel contains 0.5% tungsten (V), and 38KhNMFA steel contains 0.15% vanadium (F). The use of the latter steel is especially desirable as it contains a small amount of nickel.
Nitriding is an effective means of increasing the fatigue strength of machine parts, and it is especially effective for parts and samples with notches.
On conventional smoothly polished samples, the endurance limit as a result of nitriding increases from 49 kg/mm2 to only 52 kg/mm2, and the effectiveness of nitriding decreases with increasing diameter.
On samples with a notch in the form of an annular groove during nitriding, the endurance limit increases from 24 to 42 kg/mm2, and on samples with a notch in the form of a transverse drilling - from 14 to 29 kg/mm2.
Nitrided products do not change their structure and properties when heated to temperatures of the order of 500-600°.
Cemented parts that have a martensitic structure in the surface layer reduce their hardness when heated, starting from 220-250°. Local protection from nitriding is carried out by galvanic tinning, with the tin layer having a thickness of 0.008 mm.
Cyanidation is the process of saturating the surface layers of a product with both carbon and nitrogen. There are low-temperature and high-temperature cyanidation; the first is used for tool steels, and the second is used for strengthening structural parts.
During high-temperature cyanidation, the cement mixture contains toxic cyanide salts. Therefore, the cyanidation process must be carried out with appropriate precautions.
One common bath for high-temperature cyanidation contains 35% NaCN, 35% Na2C03 and 30% NaCl. In some cases, instead of poisonous NaCN, calcium cyanamide CaCN2, which is less toxic, is introduced into the cyanidation bath.
Parts made of both carbon and alloy steels are cyanidated. The chemical essence of the processes occurring during cyanidation is characterized by several main reactions.
In the upper layers of the cyanidation bath, where there is direct contact of molten salts with atmospheric oxygen, sodium cyanide is oxidized according to the reaction: 2NaCN + O2 = 2NaCNO. In the deeper layers of the bath, the products of the first reaction—sodium cyanate—dissociate, namely:
4NaCNO = Na2C03 + 2NaCN + CO + 2N.
The resulting carbon monoxide dissolves well in the molten salts of the bath and, upon contact with the parts in the bath, carbonizes them according to the well-known reaction: 2СО -f 3Fe = Fe3C + С02. Active atomic nitrogen, released during the dissociation of sodium cyanate, also saturates the cyanidated product.
The heat treatment of cyanidated products consists of hardening and low tempering, and exposure in a cyanidating bath is used as heating for hardening, i.e.
no additional heating required.
Low tempering is carried out in order to remove residual stresses arising during quenching and transform tetragonal martensite into cubic martensite, which is less stressed and more viscous.
The hardness of the cyanidated layer is intermediate between the hardness of the nitrided and cemented layers, since during cyanidation the saturating elements are both carbon and nitrogen.
To increase the durability of cutting tools made of high-speed steel, low-temperature cyanidation is sometimes used, carried out in a liquid or gas environment at 550-570° for 10-30 minutes as the final operation after heat treatment. For liquid cyanidation, a molten mixture of salts with the addition of K4Fe(CN)6 or NaCN is used, and for gas cyanidation, a gaseous mixture of pyrolysis products of kerosene and ammonia is used.
There is no effective protection by coatings from combined saturation with nitrogen and carbon during cyanidation. Therefore, areas that are not subject to cyanidation have to be thickened using specially left allowances, which are then removed by grinding.
What is the best cyanidation temperature?
It is important to consider many factors that will affect the operation of the device. With low-temperature cyanidation, the metal heats up at minimum levels
Hot cyanidation involves the use of baths with an average temperature of about 850 degrees.
On average, cyanidation takes up to 6 hours, so the first result is visible quite quickly. At low temperatures, less deformation occurs, so products retain their geometry and functionality. In some cases, low temperatures are not enough, so it is recommended to use hot cyanidated parts.
Alternatives to cyanide
Although cyanide is cheap, effective and biodegradable, its high toxicity has led to new methods of gold recovery using less toxic reagents. Other extractants have been tested including thiosulfate (S2O32−), thiourea (SC(NH2)2), iodine/iodide, ammonia, liquid mercury, and alpha-cyclodextrin. Challenges include reagent costs and gold recovery efficiency. Thiourea is used commercially for ores containing stibnite.[18]
The essence of technology
Cyanidation is one of the types of chemical-thermal treatment of steel. The essence of this method is to saturate metal surfaces with nitrogen and carbon in the temperature range from 530 to 950°C. In terms of technology, this resembles a combination of nitriding and carburization.
The purpose of cyanidation is to improve the properties of the metal. Thus, this processing technology increases the hardness, endurance limit, and wear resistance of the material. The principle of cyanidation is based on the diffusion of carbon and nitrogen into the structure of the material.
This process includes two stages:
- First, the top layer is saturated with carbon and nitrogen. This lasts 1 - 3 hours.
- Further, the nitrogen atoms absorbed into the structure of the material can be desorbed (exit through the surface, passing into the gas phase). At the same time, carbon saturation continues in the second stage.
The progress of the process under consideration is determined by the temperature regime. Thus, in the diffusion upper layer, as the temperature increases, the nitrogen content decreases and the amount of carbon increases, either continuously or until a specific moment. In the final stages of the operation, the nitrogen concentration begins to decrease. As a result, it is possible to record the saturation of the upper layer of steel with this element at different temperatures. The decrease in nitrogen content and increase in carbon concentration with increasing temperature occurs linearly. However, this is relevant only for the upper layer of the material, and in the underlying layers this pattern is not observed.
In addition, the characteristics of joint diffusion are affected by the amount of nitrogen, which determines the depth of carbon diffusion and the amount of saturation of the layer with it. Excessive nitrogen content may result in insufficient carbon diffusion rates. This is explained by the promotion of nitrogen to the formation of carbonitride formations on the surface.
The depth of penetration of both elements into the steel is determined by its microstructure. However, in any case, nitrogen penetrates to a greater depth than carbon.
Thus, the result of the work is determined by several factors. These include heating temperature, concentration of nitrogen and carbon, properties of the medium and material.
In-line unit for cyanidation
As a result, a two-layer coating is formed on the surface of the steel. On top there is a carbonitride layer (Fe2(C, N)) with a thickness of 10 - 15 microns. It is characterized by high wear resistance and less brittleness compared to pure nitrides and carbides. The underlying layer is represented by nitrogenous solid ferrite (martensite). Total thickness - 0.15 - 2 mm.
Kinds
Cyanidation is classified based on the following features:
- temperature conditions;
- phase composition of the medium.
Based on the phase of the medium, cyanidation is classified into:
- gas;
- hard;
- liquid.
The principle of gas cyanidation, also called nitrocarburization, is to heat an object at 530 - 570 ° C for 1.5 - 3 hours in a gas mixture containing nitrogen and carbon, including, for example, ammonia (NH3) and carbon monoxide (CO). The chemical interaction of these gases leads to the formation of atomic nitrogen and carbon. They create a layer whose thickness is determined by temperature and duration and ranges from 0.02 to 0.004 mm. Its hardness is 900 - 1200 HV.
The technology of solid cyanidation is close to hard carburization. The difference lies in the composition of the carburizer: for the work in question, a material containing cyanide salts is used. Solid cyanide plating is significantly inferior in performance to other types, so it is rarely used. Liquid and gas cyanidation are discussed below in more detail.
Cyanide plating plant
Liquid cyanidation is the most common method. In this case, molten cyanide salts are used, represented by NaCl, NaCN, Na2CO3, BaCl2, BaCO2 in various concentrations and combinations.
There are regulations that determine the temperature regime and duration of work for different mixture compositions. It also displays the thickness of the resulting layer, which is 0.15 - 1.6 mm. The interaction of sodium cyanide salts with soda and salt leads to their decomposition with the release of atomic nitrogen and carbon. The main component of cyanide salts is CN. An increase in its content leads to an increase in the concentration of nitrogen and carbon in the diffusion layer, but does not affect its thickness. Liquid cyanidation serves as a finishing treatment for steel.
Based on the temperature regime, cyanidation is divided into low and high temperature. Treatment of the first type of metal provides greater saturation with nitrogen, and high-temperature cyanidation - on the contrary, with carbon.
Liquid high-temperature treatment, also called liquid carburization, is carried out by keeping parts in bath furnaces at 840 - 950 ° C for 5 - 45 minutes. This method allows you to achieve a diffusion layer thickness of up to 0.075 - 0.1 mm. This parameter is determined by the temperature and duration of the process. In any case, building up a layer using this method is faster than using gas cyanidation. However, this method is very harmful, since molten cyanide salts are toxic. Therefore, special safety measures are required when carrying out such work.
In view of this, gas cyanidation is preferred to liquid high-temperature technology, despite the lower speed of work. This is compensated by the lower cost. It is carried out at 830 - 950 ° C in muffle furnaces for 1 - 2 hours. Upon completion of hardening and low tempering, the hardness of the material processed by this method increases to 60 - 64 HRC (56 - 62 according to other data).
Low-temperature cyanidation of medium-carbon steel is also called the tenifer process. It consists of saturating the material predominantly with nitrogen by passing dry air through it at 540 - 600°C.
Before low-temperature cyanidation, a full cycle heat treatment is carried out at 500 - 600°C.
Steel cyanidation process
Thus, low-temperature cyanidation creates a layer with a high nitrogen content, and at high temperature a coating of predominantly carbon composition is formed (carbon concentration is 0.6 - 1.2%, nitrogen - 0.2 - 0.6%).
Purpose of the process
Normalization is designed to change the microstructure of steel; it does the following:
- reduces internal stress;
- through recrystallization, it refines the coarse-grained structure of welds, castings or forgings.
The goals of normalization can be completely different. Using this process, the hardness of steel can be increased or decreased, the same applies to the strength of the material and its toughness. It all depends on the mechanical and thermal characteristics of the steel. Using this technology, it is possible to both reduce residual stresses and improve the degree of machinability of steel using one or another method.
Steel castings are subjected to this treatment for the following purposes:
- to homogenize their structure;
- to increase susceptibility to heat hardening;
- to reduce residual stresses.
Products obtained by forming are subjected to normalization after forging and rolling in order to reduce the heterogeneity of the structure and its banding.
Normalization together with tempering is needed to replace the hardening of products with complex shapes or with sharp changes in cross-section. This will prevent defects.
This technology is also used to improve the structure of the product before hardening, increase its machinability through cutting, eliminate the secondary cement network in hypereutectoid steel, and also prepare the steel for final heat treatment.
Related methods
A similar method is considered to be soft nitriding. It is performed at a temperature of about 590°C. This treatment is used to increase the wear resistance and endurance limit of medium-carbon steels.
Also, in terms of technology, the treatment in question is close to cementation. Compared to cyanidation, cyanidation stands out in that the layer formed has better wear resistance and corrosion resistance, greater hardness, and fatigue strength. Moreover, due to lower temperatures and duration of the process, grain growth does not occur. In view of this, immediately after cyanidation is completed, hardening is performed, which gives the surface greater hardness. Finally, the high-temperature cyanidation process of steel takes less time than carburizing.
If you find an error, please select a piece of text and press Ctrl+Enter.
Types of metal that can be processed
There are three main groups of metal used for hardening:
- Steel with non-hardening core. This group includes the following grades of steel suitable for cementing - 20, 15 and 10. These parts are small in size and are used for use in domestic conditions. During hardening, austenite is transformed into a ferrite-pearlite mixture.
- Steel with a weakly hardened core. This group includes metals of such grades as 20Х, 15Х (low-alloy chromium steels). In this case, an additional ligation procedure is performed using small doses of vanadium. This ensures a fine grain, which results in a more ductile and ductile metal.
- Steel with a highly hardened core. This type of metal is used for the manufacture of parts with complex configurations or large cross-sections that can withstand various shock loads and are exposed to alternating current. During the hardening process, nickel is introduced or, if it is deficient, manganese is used, while small doses of titanium or vanadium are added to crush the grain.
Chemical reactions
Balloon model of the complex anion aurocyanide or dicyanoaurate (I), [Au(CN)2]−.[10]
Cyanide leaching "pile" at a gold mining operation near Elko, Nevada The chemical reaction for dissolving gold, the "Elsner equation", is as follows:
4 Au (s) + 8 NaCN (aq) + O2 (g) + 2H2O (l) → 4 Na (aq) + 4 NaOH (aq)
In this, redox oxygen removes, through a two-step reaction, one electron from each gold atom to form an Au(CN)− 2 ion complex.[11]
The purpose of steel cyanidation and the essence of technology
The primary goal of cyanidation is to strengthen the surface layer of steel of various parts, giving it a higher endurance limit, since this layer is subject to the greatest loads during operation of mechanisms and structures. Saturation of the surface layer of the metal with carbon and nitrogen is usually used due to their rapid penetration when they interact simultaneously. The following types of metal can be processed using cyanidation:
- any stainless steel;
- alloyed steel alloys or those without the presence of alloying components, steels with an average carbon concentration;
- steel for structural purposes, where there is little carbon present.
The chemical-thermal cyanidation method adheres to the following technology:
- The part is placed in a working bath with molten cyanide salt of the composition 15% Na₂CO₃, 60% NaCl and 25% NaCN.
- Next, the working medium is heated to a temperature from 930 to 530 degrees Celsius (depending on the selected processing mode).
- Carbon monoxide and nitrogen released from the salt saturate the metal for several hours.
The essence of the process by which carbon and nitrogen can penetrate into a layer of steel is diffusion. During the technology stages listed above, the process goes through two main stages, separated by time periods:
- The initial period of nitrocarburization lasting from one to three hours, characterized by the introduction of nitrogen and carbon atoms into the crystal lattice of the metal.
- The final period when nitrogen atoms that have previously penetrated and saturated the steel begin to desorb (leave the surface, again acquiring the state of a gas), while carbon continues to saturate the metal until the effect of temperature and working environment ends.
How to prepare a part
The surfaces of the part must be cleaned and degreased before nitrocarburizing. To do this, it is enough to wash them for 15 minutes in a solution of caustic soda heated to 90 ° C, or you can wipe them with gasoline. Then the parts are wiped dry and placed in baskets at a distance sufficient for free penetration of gas.
What can be saturated with carbon?
It is advisable to carry out nitrocarburization with stainless steel, alloys containing alloying additives, and structural steels with low carbon content.
Main defects during nitrocarburization
During the nitrocarburizing process, defects in the processed parts may occur.
Peeling
This phenomenon occurs when the surface of the part is saturated with carbon and is associated with too low temperatures or rapid heating. In the first case, the carbon content levels out too slowly towards the center. With rapid heating, the carbon content decreases sharply with distance from the surface of the part. Such sudden changes provoke separation of the cemented layer from the product in the form of peeling of the shell.
Coarse-grained fracture
The coarseness of the treated layer can be caused by several factors: overheating, overexposure during hardening, excess carbon in the cemented layer due to high or changing temperatures during processing. These defects can be eliminated by re-hardening. Coarse graining of the core can occur due to the quenching temperature being too low. And if we are talking about low-alloy or carbon steels, then this defect can be explained by the parts being too large, which does not allow the core to be sufficiently calcined.
Soft surface
This defect in the surface of processed products is caused by a number of violations of the nitrocarburization process (the appearance of voids when stuffing parts, the appearance of a graphite crust on the surface of the part). Such a defect can also be caused by a hardening defect associated with a low cooling rate or the formation of a steam jacket. When nitriding, soft spots are associated with the processing of non-greased parts.
Low saturated film thickness
This defect occurs at low nitriding temperatures. The flaw is extremely dangerous, since it is impossible to detect it using conventional control methods. But the problem can be eliminated by repeating the procedure while observing the temperature regime.
Increased fragility
Associated with nitriding of a decarbonized surface. The latter is formed on the part during thermal or hot pressure treatment. This layer must be mechanically removed.
The hardness of the nitrided surface is slightly lower than the hardness of the layer immediately below the surface. When processing highly loaded parts in this way, it is necessary to sand the top layer, thereby removing it.
Nitriding of steel
When nitriding, the surface layer of a steel part is saturated with oxygen. This method received industrial application almost 100 years ago, in the 20s of the 20th century. Nitriding a part is an excellent way to increase not only the hardness of the product, but also its corrosion resistance.
Nitriding of steel is carried out by immersing the part in furnaces, which are hermetically sealed. Ammonia is supplied there, which, when heated, breaks down into nitrogen and hydrogen. During this reaction, nitrogen atoms are absorbed by the steel surface layer and penetrate into the part.
Statement
The ore is crushed using grinding equipment. Depending on the ore, it is sometimes further concentrated using froth flotation or. Water is added to make a paste
or
pulp
. The base ore slurry may be combined with a solution of sodium cyanide or potassium cyanide; many operations use calcium cyanide, which is more economical.
To prevent the creation of toxic hydrogen cyanide during processing, slaked lime (calcium hydroxide) or soda (sodium hydroxide) is added to the extractant solution to ensure that the acidity during cyanidation is maintained over a pH of 10.5 – highly alkaline. Lead nitrate can improve gold leaching rates and quantities, especially when processing partially oxidized ores.
Effect of dissolved oxygen
Oxygen is one of the reactants consumed during cyanidation by accepting electrons from the gold, and a lack of dissolved oxygen reduces the rate of leaching. Air or pure oxygen gas can be blown through the pulp to maximize dissolved oxygen concentration. Internal oxygen-pulp contactors are used to increase the partial pressure of oxygen contacting the solution, thus raising the dissolved oxygen concentration well above the saturation level at atmospheric pressure. Oxygen can also be added by dosing the pulp with a solution of hydrogen peroxide.
Pre-aeration and ore washing
In some ores, especially partially sulfided ones, aerating (before introducing cyanide) the ore into water at high pH can make elements such as iron and sulfur less reactive to cyanide, making the gold cyanidation process more efficient. In particular, the oxidation of iron to iron(III) oxide and subsequent precipitation as iron hydroxide minimizes the loss of cyanide due to the formation of ferrous cyanide complexes. Oxidation of sulfur compounds to sulfate ions avoids the conversion of cyanide to the byproduct thiocyanate (SCN – ).
Story
In 1783, Karl Wilhelm Scheele discovered that gold dissolves in aqueous solutions of cyanide. Thanks to the work of Bagration (1844), Elsner (1846) and Faraday (1847), it was determined that for each gold atom one cyanide ion was required, that is, the stoichiometry of the soluble compound.
Industrial process
John Stewart MacArthur developed the cyanide process to extract gold in 1887.
The expansion of gold mining in the Rand of South Africa began to slow in the 1880s, as new deposits were discovered, usually pyrite ores. Gold could not be extracted from this compound by any of the chemical processes or technologies then available. In 1887, John Stuart MacArthur, working in collaboration with brothers Robert and William Forrest for the Tennant Company in Glasgow, Scotland, developed the MacArthur–Forrest process for extracting gold from gold ores. Several patents were issued that year. By suspending crushed ore in a cyanide solution, separation of up to 96% pure gold was achieved. The process was first used at Rand in 1890 and, despite operational shortcomings, led to a boom in investment as larger gold mines were opened.
By 1891, Nebraska pharmacist Gilbert S. Peyton had perfected the process at his Mercure Mine in Utah, "the first mining operation in the United States to achieve commercial success with the cyanide process on gold ores." In 1896, Bodlender confirmed that oxygen was needed for this process, which MacArthur had doubted, and discovered that hydrogen peroxide was formed as an intermediate product. Around 1900, American metallurgist Charles Washington Merrill (1869-1956) and his engineer Thomas Bennett Crowe improved the purification of cyanide leachate using vacuum and zinc dust. Their process is the Merrill–Crowe process.
Steel Cementation
Cementation is a process that saturates a steel structure with carbon. The core remains soft, however, thanks to the coating layer, the surface strength increases. During use, such parts are not exposed to external influences, are not deformed by impacts and are not erased.
Cementation is applied to elements made of carbon or alloy steel, the carbon content of which is not less than 0.08% and not more than 0.35%. Carbon-rich compounds are used for cementation. They are called carburizers. Such compositions can be liquid, solid and even gaseous.
Cementation of steels occurs through heating of parts pre-packed in boxes made of iron, and a carburizer is also placed there. The solid consists of 70% charcoal, 20–25% barium carbonate, and the remainder calcium carbonate (3–5%).
Cementation is carried out at a temperature of 920–930 °C, this indicator allows you to make the process as fast as possible. Enrichment of the steel layer occurs when coal particles come into contact with the surface of the element. The carbon transmitter in this situation is the gaseous medium. Properly organized cementation of the surface layer of a steel part lasts from 5 to 14–15 hours.
It is customary to subject small-sized products made of carbon or alloy steel to cementation in a liquid medium. They are dipped for some time in salt baths that contain molten substances:
- soda;
- table salt;
- silicon carbide.
Steel carburization scheme
Gas cementation
The essence of gas carburization is that an alloy steel part must first be heated and then calcined in a furnace, the temperature in which is from 920 to 950 °C. Gas containing methane is supplied to the furnace chamber throughout the entire carburization period.
When using this method, the duration of carburization of a steel part is reduced several times. Thus, the depth of the cementation layer of 1.2 m can be recorded after 4–5 hours of the part being in the gas chamber.
Gas carburization of steels has clear advantages over the first two methods:
- the ability to regulate the process by changing the quantitative and qualitative composition of the gas;
- lack of large equipment;
- relative purity of the process, absence of coal dust;
- the ability to harden steel directly in the furnace chamber.
Gas carburization is quite economical compared to the use of solid and liquid carburizers.
Related methods
A similar method is soft nitriding. It is carried out at a temperature of approximately 590°C. This treatment is used to increase the wear resistance and endurance limit of medium-carbon steels.
Also, in terms of technology, the treatment in question is close to cementation. In comparison with cyanidation, cyanidation differs favorably in that the resulting layer has better wear resistance and corrosion resistance, greater hardness, and fatigue strength. In addition, due to lower temperature conditions and process duration, grain growth does not occur. In view of this, immediately after cyanidation is completed, hardening is carried out, which gives the surface greater hardness. Finally, the high-temperature cyanidation process of steel takes less time than carburizing.
Source
Legislation
See also: Ban on cyanidation of gold
The US states of Montana[19] and Wisconsin,[20] the Czech Republic,[21] Hungary,[22] have banned cyanide mining. The European Commission rejected the proposal for such a ban, noting that existing rules (see below) provide adequate protection of the environment and health.[23] Several attempts to ban gold cyanidation in Romania have been rejected by the Romanian Parliament. There are currently protests in Romania calling for a ban on the use of cyanide in mining (see 2013 Romanian protests against the Roșia Montană project).
In the EU, the industrial use of hazardous chemicals is controlled by the so-called Seveso II Directive (Directive 96/82/EC,[24] which replaced the original Seveso Directive (82/501/EEC[25] brought after the dioxin disaster of 1976. "Free cyanide and any compound capable of releasing free cyanide in solution" are further controlled by inclusion in Schedule I of the Groundwater Directive (Directive 80/68/EEC)[26] which prohibits any discharge of such a size as is likely to cause deterioration of groundwater quality now or in the future The Groundwater Directive was largely replaced in 2000 by the Water Framework Directive (2000/60/EC).[27]
In response to the Baia Mare cyanide spill in 2000, the European Parliament and Council adopted Directive 2006/21/EC on the management of waste from extractive industries.[28] Article 13(6) requires that "the concentration of weakly acidic dissociable cyanide in the pond be reduced to the lowest possible level using the best available techniques" and in most cases all mines operating after 1 May 2008 cannot discharge waste containing more than 10 ppm cyanide WAD, mines built or permitted before this date initially allow no more than 50 ppm, decreasing to 25 ppm in 2013 and 10 ppm by 2022.
Under Article 14, companies must also provide financial guarantees to ensure cleanup after the mine is completed. This may in particular impact smaller companies wishing to build gold mines in the EU, as they are less likely to have the financial capacity to provide such guarantees.
Industry came up with a voluntary “ Cyanide Code”
“[29] which aims to reduce environmental impacts through third-party audits of the company's cyanide management.
Recommendations
- Roubo, Andreas; Kellens, Raf; Reddy, Jay; Steyer, Norbert; Hasenpusch, Wolfgang (2006). "Alkali metal cyanides". Ullman Encyclopedia of Industrial Chemistry
. Doi:10.1002 / 14356007.i01_i01. ISBN 978-3527306732. - Barrick Gold - Cyanide Facts Archived 2010-09-20 at the Wayback Machine
- ^ a b
Gray, J. A.;
McLachlan, J. (June 1933). "The History of the Introduction of the MacArthur-Forrest Cyanide Process to the Witwatersrand Goldfields". Journal of the South African Institute of Mining and Metallurgy
.
33
(12): 375–397. HDL:10520 / AJA0038223X_5033. - us US403202, MacArthur, John Stewart; William Forrest and Robert Forrest Robert, "The Process of Obtaining Gold and Silver from Ores," issued May 14, 1889.
- "Gold Extraction Methods II". 2013-05-14.
- ^ a b
Habashi, Fathi Recent advances in gold metallurgy Archived 2008-03-30 on the Wayback Machine - Quarterly and Bi-Weekly Alumni Notes
. University of Illinois. January 1, 1921. Retrieved May 1, 2016. - "Mercury, Utah." Retrieved May 1, 2016.
- Adams, Mike D. (2005-12-02). Advances in gold ore processing
. Elsevier. pp. XXXVII – XLII. ISBN 978-0-444-51730-2. ISSN 0167-4528. - Greenwood, N.N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.), Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- (Internet Archive) Technical Bulletin, https://web.archive.org/web/20091023235047/https://www.multimix.com.au/DOCUMENTS/Technical%20Bulletin1.PDF
- ^ a b
UNEP/OCHA Environment Division "UN Assessment Mission - Baia Mare Cyanide Spill, March 2000" - Maprani, Antu C.; Al, Tom A.; MacQuarrie, Kerry T.; Dalziel, John A.; Shaw, Sean A.; Yates, Philip A. (2005). “Determining Mercury Evasion in a Contaminated Upstream.” Environmental Science and Technology
.
39
(6):1679–1687. Bibcode:2005EnST...39.1679M. Doi:10.1021/es048962j. PMID 15819225. - Al, Tom A.; Leybourne, Matthew I.; Maprani, Antu C.; MacQuarrie, Kerry T.; Dalziel, John A.; Fox, Don; Yates, Philip A. (2006). "The influence of acid-sulfate weathering and cyanide-bearing gold tailings on the transport and fate of mercury and other metals in Gossan Creek: Murray Brook Mine, New Brunswick, Canada." Applied Geochemistry
.
21
(11): 1969–1985. Bibcode:2006ApGC...21.1969A. doi:10.1016/j.apgeochem.2006.08.013. - "Long-term persistence of cyanide in the mine waste environment", B. Yarar, Colorado School of Mines, Tailings and Mine Waste '02, Swets & Zeitlinger ISBN 90-5809-353-0, p.197 (Google Books)
- BBC News BBC: "Cyanide seeps into PNG rivers" 23 March 2000
- Wilson, T. La politica es la politica: "Can First Majestic Clean Up Cyanide Spills After a Spill?" April 21, 2018.
- La Brooy, S. R.; Linge, H.G.; Walker, G.S. (1994). "Review of gold mining from ores." Mineral Engineering
.
7
(10): 1213–1241. Doi:10.1016/0892-6875(94)90114-7. - Citizens' Initiative Bans Cyanide Mining in Montana, USA Archived October 21, 2007 Wayback Machine
- Senate Bill 2001 160 regarding the use of cyanide in the mining industry.
- “The Czech Senate bans the use of cyanide in gold mining.” Nl.newsbank.com. 2000-08-10. Retrieved 2013-01-03.
- Zöld siker: törvényi tilalom a cianidos bányászatra! Archived July 21, 2011 Wayback Machine
- International Mining - European Commission rejects proposed ban on cyanide in mining, July 2010
- Council Directive 96/82/EC of 9 December 1996 on the control of the hazards of major accidents involving hazardous substances. For changes, see summary version.
- Council Directive 82/501/EEC of 24 June 1982 on the hazards of major accidents in certain industrial activities. Not valid.
- Council Directive 80/68/EEC of 17 December 1979 on the protection of groundwater against pollution caused by certain hazardous substances. Not valid.
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy ( Water Framework Directive
). For changes, see summary version. - Directive 2006/21/EC of the European Parliament and of the Council of 15 March 2006 on the management of waste from extractive industries. For changes, see summary version.
- ICMI www.cyanidecode.org International Code for the Management of Cyanide in the Production, Transport and Use of Cyanide in Gold Production