Chemistry is an amazing science. So many incredible things can be found in seemingly ordinary things. Everything material that surrounds us everywhere exists in several states of aggregation: gases, liquids and solids. Scientists have also identified the 4th - plasma. At a certain temperature, a substance can change from one state to another. For example, water: when heated above 100, from liquid form it turns into steam. At temperatures below 0, it transforms into the next aggregate structure - ice.
…
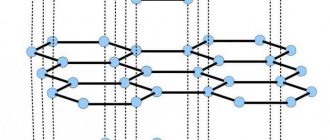
The entire material world contains a mass of identical particles that are interconnected. These smallest elements are strictly lined up in space and form the so-called spatial frame.
This is interesting: anions and cations in chemistry, solubility table.
Definition
A crystal lattice is a special structure of a solid substance in which the particles stand in a geometrically strict order in space.
In it you can find nodes - places where elements are located: atoms, ions and molecules and internodal space. Solids , depending on the range of high and low temperatures, are crystalline or amorphous - they are characterized by the absence of a certain melting point. When exposed to elevated temperatures, they soften and gradually turn into liquid form. These types of substances include: resin, plasticine.
This is interesting: hydrogen bond - examples, mechanism of formation.
In this regard, it can be divided into several types:
- atomic;
- ionic;
- molecular;
- metal.
But at different temperatures, one substance can have different forms and exhibit diverse properties. This phenomenon is called allotropic modification.
This is interesting: metals and non-metals in the periodic table of Mendeleev.
MORPHOLOGY
Well-formed crystals are rare. The crystals are lamellar, scaly, curved, usually of an imperfect lamellar shape. Most often these are leaves without crystallographic outlines and their aggregates. Forms continuous radial-radial aggregates, cryptocrystalline, sheet or round, less often - spherulite aggregates of concentric-zonal structure. In coarse-crystalline sediments, triangular shading is often observed on the (0001) planes.
Ionic type
Oppositely charged ions are located at nodes that create an electromagnetic field that characterizes the physical properties of a substance. These will include: electrical conductivity, refractoriness, density and hardness. Table salt and potassium nitrate are characterized by the presence of an ionic crystal lattice.
Don't miss: the mechanism of metal bond formation, specific examples.
ORIGIN
It forms at high temperatures in volcanic and igneous rocks, pegmatites and skarns. It occurs in quartz veins with wolframite and other minerals in mid-temperature hydrothermal polymetallic deposits. Common in metamorphic rocks - crystalline schists, gneisses, marbles. Large deposits are formed as a result of coal pyrolysis under the influence of traps in coal deposits (Tunguska basin). Accessory mineral of meteorites. Associated minerals: quartz, pyrite, garnets, spinel.
Metal type
Its structure resembles a molecular one, but it still has stronger bonds. The difference between this type is that its nodes contain positively charged cations. Electrons that are located in the interstitial space participate in the formation of the electric field.
They are also called electric gas. Simple metals and alloys are characterized by a metal lattice type. They are characterized by the presence of a metallic luster, plasticity, thermal and electrical conductivity. They can melt at different temperatures.
| Kinds | Substances | Properties |
| Nuclear | Diamond, graphite, silicon, boron | Hard, refractory, insoluble in water |
| Molecular | Iodine, sulfur, white phosphorus, organic matter | Non-solid, melts easily, volatile |
| Ionic | Salts, oxides and hydroxides of heavy metals | Hard, brittle, fusible, electrically conductive |
| Metal | Metals and alloys | Shiny, malleable, thermally and electrically conductive. |
Properties of graphite:
– the electrical conductivity of graphite is anisotropic (that is, it depends on the direction inside the graphite itself). It conducts electric current well in a direction parallel to the base plane. In this case, its electrical conductivity is close to metallic. In the perpendicular direction, electrical conductivity is hundreds of times less.
– has low hardness. Mohs hardness 1.
– relatively soft. After exposure to high temperatures it becomes slightly harder and very brittle,
– density 2.08-2.23 g/cm³,
– easy to machine,
– color from iron-black to steel-gray, metallic luster,
– infusible, stable when heated in the absence of air,
– greasy (slippery) to the touch, leaves a mark on paper and fingers,
– during friction, graphite exfoliates into separate flakes (this property is used in pencils),
– has a fairly high thermal conductivity. The thermal conductivity of graphite is anisotropic. It ranges from 100 to 354.1 W/(m*K) and depends on the grade of graphite, on the direction relative to the basal planes and on temperature,
– the coefficient of thermal expansion of graphite is also anisotropic and depends on temperature. Up to 700 K, the coefficient of thermal expansion of graphite is negative in the direction of the basal planes (graphite contracts when heated), its absolute value decreases with increasing temperature. Above 700 K the coefficient of thermal expansion becomes positive. In the direction perpendicular to the basal planes, the coefficient of thermal expansion is positive, practically independent of temperature and more than 20 times higher than the average absolute value for the basal planes,
– has high diamagnetism,
– chemically inactive,
– has chemical resistance. Acid resistant
– at high temperatures reacts with oxygen, burning to carbon dioxide,
– forms inclusion compounds with alkali metals and salts.
Various substances
- Diamond. The mineral is of high value and, after cutting, is used in jewelry. So what is the secret of the popularity of this stone? Carbon atoms form the basis of the entire lattice. There is a strong covalent bond between the atoms of the mineral. The crystal lattice of diamond is characterized by a dense content of atoms in the form of a cube. In other words, carbon atoms are considered nodes, and the peculiar faces of the cube are strong covalent bonds. This mineral is considered the most durable on the planet, and it is unknown how many of these unique cubes include a solid diamond.
- Graphite. Carbon can also be in another crystalline modification. The atomic lattice of this element includes only carbon atoms and has a layered structure. In graphite, each atom is bonded by three carbon atoms. Because of this, it has a metallic luster and high thermal conductivity.
- The crystal lattice of iodine is of a molecular type. Atoms of molecules are connected by covalent bonds, but the molecules of a chemical element have weak attractive forces. This characterizes iodine in that it has low hardness and a low melting point.
- Sodium. Representative of a metal crystal lattice. Electrons move between cations located at lattice sites. By joining cations, they neutralize their charge, in turn, neutral atoms release some electrons, transforming into cations. This type of crystal lattice gives the metal plasticity, electrical and thermal conductivity.
- Dry ice. Or carbon monoxide in solidified form. It has a molecular crystal lattice in the shape of a cube. Molecules are held together by weak bonds. infusion, read our article.
This is interesting: how to determine valency using the periodic table?
Description of graphite:
Graphite (translated from Greek as “I write”) is a natural material belonging to the class of native elements, an allotropic modification of carbon. The chemical formula of graphite is C.
Along with graphite and diamond, there are many more allotropic forms of carbon. For example, graphene, fullerene, carbon nanotubes, etc. The properties of these substances are completely different from each other.
Graphite is widely distributed in nature as a mineral. It is usually found in the form of individual scales, plates and clusters, varying in size and content.
There are deposits of crystalline graphite associated with igneous rocks or crystalline shales, and cryptocrystalline graphite formed during the metamorphism of coals.
Natural graphite is not pure in its chemical composition. It contains large quantities (up to 10-25%) of ash, consisting of various components (Fe2O3, SiO2, Al2O3, MgO, P2O5, CuO, CaO, etc.), gases (up to 2%) and bitumen, and sometimes water.
Graphite is also obtained artificially in various ways. For example, by heating a mixture of coke and pitch to 2,800 °C.
Therapeutic effect
Graphite has a large number of healing properties, which allows folk medicine to actively use it as a remedy for diseases.
- Graphite has a beneficial effect on the layers of the epidermis. It is used in the treatment of skin cracks, scars, bruises, and eczema.
- Lithotherapists use graphite products to treat diseases in the sinus area. The stone helps get rid of dryness in the nasal mucosa, eliminate various chronic diseases of the respiratory tract, rhinitis, laryngotracheitis. Graphite is also used as a prophylactic treatment for bronchial asthma.
- Graphite stones help improve the condition of the gastrointestinal tract, improve metabolic processes in the body, fight chronic gastritis, eliminate heartburn and reduce the worsening of constipation or diarrhea.
- Healing graphite helps cope with severe headaches, apathy and stress.
- Graphite has a healing effect on human eyes. Small stones relieve inflammation in barley, conjunctivitis, and also help in the treatment of cataracts or corneal ulcers.
- The mineral regulates emotional balance, reducing the level of anger, temper, aggression, depression and neurasthenia.
Graphite has a crystal lattice
The table below lists the characteristic properties of substances with atomic and ionic crystal lattices.
Characteristic properties of substances
- solid under normal conditions;
- conduct electric current in melts and solutions
| With atomic crystal lattice | With ionic crystal lattice |
Using this information, determine what kind of crystal lattice it has:
1) calcium chloride
;
2) graphite
.
Write your answer in the space provided:
1) Calcium chloride has
Calcium chloride is a substance with an ionic chemical bond, refractory (T mp = 772 °C), conducts electric current - has an ionic crystal lattice.
Graphite is a substance with a covalent nonpolar chemical bond, nonvolatile, solid, and has an atomic crystal lattice.
Answer: Calcium chloride is an ionic crystal lattice, graphite is an atomic crystal lattice.
Source
Place of Birth
Graphite mine
The demand for graphite in industry is very high. Today, approximate reserves around the world are estimated at 600 million tons. The largest graphite deposits are located in China, Mexico, Russia, the Czech Republic, South Korea and other countries. In addition to the countries listed, graphite is also mined on the island of Sri Lanka. Large reserves of this mineral were also found in Ukraine, in the so-called Zavalevskoe deposit. The discovered graphite deposits are industrially significant and are in great demand.
Where and how is it mined?
Graphite is mined all over the world. The ranking of countries by reserves and production in the world is presented below.
- China . About 780 thousand tons per year. In 2022, the country became the largest producer of this mineral in the world. During the year, China produced the same amount of graphite as in the previous 2 years. The US Geological Survey estimates that the country accounts for 65% of global production and 35% of consumption.
- India. 150 thousand tons were produced. Deposit reserves vary from state to state. 43% of the reserves are in Arunachal Pradesh. In total, there are 8 graphite mining and production companies in the country.
- Brazil . Production is 90 thousand tons per year. All leading manufacturing companies are private. The production level remains the same, but there are prerequisites for companies from China and India to squeeze them out of the market.
- Canada . 30 thousand tons of graphite are mined per year. Demand for Canadian graphite is growing, especially after Tesla announced it would purchase graphite for battery production from companies based in Canada.
- Mozambique . Production 23 thousand tons. Graphite mining in this country has grown from zero in 2016 to present in 2022. The pace of production is constantly increasing, the market in this region is represented by two companies.
Other countries produce less than 20 thousand tons per year. The top ten countries are rounded out by Russia (6th place), Ukraine, Pakistan, Norway and Madagascar. In total, these countries mine about 63 thousand tons of graphite per year. The graphite market in the world is constantly growing. This is due to the transition to electric batteries in many industries. The surge in electric vehicle production around the world is stimulating demand for graphite.
World consumption grew from 2013 to 2022, then there was a decrease in demand for this mineral. Prices dropped to 2017 levels as there was an oversupply and companies reduced production to stabilize world prices for the mineral.
APPLICATION
For the manufacture of melting crucibles, lining plates - the application is based on the high heat resistance of graphite (in the absence of oxygen), its chemical resistance to a number of molten metals. It is used in electrodes and heating elements due to its high electrical conductivity and chemical resistance to almost all aggressive aqueous solutions (much higher than that of precious metals). For the production of chemically active metals by electrolysis of melts, solid lubricants, combined liquid and paste lubricants, plastic fillers.
it is a neutron moderator in nuclear reactors, a component of a composition for the production of rods with black graphite (mixed with kaolin). It is used to obtain synthetic diamonds as a nanometer length standard for the calibration of tunnel effect microscope and atomic force microscope scanners, for the manufacture of contact brushes and current collectors for various electrical machines, electric vehicles and trolley overhead cranes, high-power rheostats and other devices where required reliable mobile electrical contact for the manufacture of thermal protection for the nose of ballistic missile warheads and re-entry spacecraft.
STRUCTURE
Hexagonal crystalline polymorphic (allotropic) modification of pure carbon, the most stable under the conditions of the earth's crust.
The layers of the crystal lattice can be differently positioned relative to each other, forming a whole range of polytypes, with symmetry from hexagonal system (dihexagonal-dipyramidal type of symmetry) to trigonal (ditrigonal-scalenohedral symmetry). The crystal lattice of graphite is layered. In layers, C atoms are located at the sites of hexagonal cells of the layer. Each C atom is surrounded by three neighboring atoms with a distance of 1.42Α. There are two modifications of graphite: α-graphite (hexagonal P63/mmc) and β-graphite (rhombohedral R(-3)m). They differ in the packing of layers. In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon (laying...AVAVAVA...), and in β-graphite, every fourth layer repeats the first. It is convenient to represent rhombohedral graphite along hexagonal axes to show its layered structure.
β-graphite is not observed in its pure form, since it is a metastable phase. However, in natural graphites the content of the rhombohedral phase can reach 30%. At a temperature of 2500-3300 K, rhombohedral graphite completely transforms into hexagonal graphite.