Why gas burns with a red flame on a stove: factors influencing the color of the flame Over time, many people notice how the color of the flame changes from blue to some other color. The question arises: what color should the gas be on the stove during normal operation of the equipment? Is it normal that it lights up not blue, but some other color? And in what cases does a change in flame color indicate serious problems.
Indeed, a change in flame color almost always indicates problems. But most of them can be solved independently and without even calling special services. Let's look at why gas sometimes burns with an orange rather than a blue flame, and how to fix this problem.
Publications on the Internet explain differently how the color of a fire's flame appears.
There are two fundamentally different versions.
One says that they emit incandescent carbon particles about 100 nm in size, the second says that the yellow color arises from the emission of sodium salts found in wood. Numerous publications have given one or the other of these explanations. This topic is discussed on forums, but no one refers to the results of experiments.
Here is an example of typical publications:
That is, until now there is no generally accepted explanation for the mechanism of visible radiation that occurs during the burning of a fire!
Description:Wetting a copper plate in hydrochloric acid and bringing it to the burner flame, we notice an interesting effect - coloring of the flame. The fire shimmers with beautiful blue-green shades. The spectacle is quite impressive and mesmerizing.
Copper gives the flame a green tint. With a high copper content in the combustible substance, the flame would have a bright green color. Copper oxides give an emerald green color. For example, as can be seen from the video, when copper is wetted with hydrochloric acid, the flame turns blue with a greenish tint. And calcined copper-containing compounds soaked in acid color the flame azure blue.
For reference: Green color and its shades are also given to fire by barium, molybdenum, phosphorus, and antimony.
Explanation:
Why is the flame visible? Or what determines its brightness?
Some flames are almost invisible, while others, on the contrary, shine very brightly. For example, hydrogen burns with an almost completely colorless flame; the flame of pure alcohol also shines very weakly, but a candle and a kerosene lamp burn with a bright luminous flame.
The fact is that the greater or lesser brightness of any flame depends on the presence of hot solid particles in it.
Fuel contains carbon in greater or lesser quantities. Carbon particles glow before they burn, which is why the flame of a gas burner, kerosene lamp and candle shines - because it is illuminated by hot carbon particles.
Thus, it is possible to make a non-luminous or weakly luminous flame bright by enriching it with carbon or heating non-combustible substances with it.
How to get multi-colored flames?
To obtain a colored flame, not carbon is added to the burning substance, but metal salts that color the flame in one color or another.
The standard method of coloring a faintly luminous gas flame is to introduce metal compounds into it in the form of highly volatile salts - usually nitrates (salts of nitric acid) or chlorides (salts of hydrochloric acid):
yellow – sodium salts,
red – strontium, calcium salts,
green – cesium salts (or boron, in the form of boronethyl or boronmethyl ether),
blue – copper salts (in the form of chloride).
Selenium colors the flame blue, and boron colors the flame blue-green.
This ability of burning metals and their volatile salts to impart a certain color to a colorless flame is used to produce colored lights (for example, in pyrotechnics).
What determines the color of a flame (in scientific language)
The color of a fire is determined by the temperature of the flame and what chemicals it burns. The high temperature of the flame allows atoms to jump to a higher energy state for some time. When the atoms return to their original state, they emit light at a specific wavelength. It corresponds to the structure of the electronic shells of a given element.
Homemade spectrometer
There are many publications and videos on the Internet about how to make a spectrometer from a DVD, but the characteristics of these devices do not allow you to carry out the necessary measurements. I managed to make a high-quality spectrometer.
Main characteristics
The spectrometer operates in the range of 400-700 nm with a resolution of 0.3 nm. Replaceable optical slits with widths of 50, 100, 200 and 300 microns are used. The diffraction grating with a pitch of 740 nm is made of a DVD disc. The spectrum is recorded using a Nikon D5100 SLR camera. The device is made in a durable case that allows you to save settings when moving.
Measuring the spectrum of a fire flame
Classic experiments were carried out - the spectra of the Sun, lasers, gas burner flames and all kinds of lamps were measured. The spectrometer passed the test and now we could begin to study the fire flames.
Examined fire flame in the fireplace
I lit a fire in the fireplace and conducted research, recording the spectrum of the flame
Let's measure the spectrum of the line of fire - that's what I called the line I saw.
Against the background of a very weak continuous black-body spectrum, two bright yellow lines were detected with wavelengths of 589.0 nm and 589.6 nm. According to the NIST database, these are sodium lines.
Spectra of a calibration lamp, fire in the fireplace, table salt and ash from the fireplace
The photograph below shows part of the spectrum of a fire flame at high magnification so that the double line of sodium 589.0 nm and 589.6 nm can be seen against the background of a continuous spectrum of incandescent carbon particles:
Close-up of spectral lines of sodium in a fire and sodium lines in ash burning in alcohol.
In further studies, the dynamics of the appearance of sodium lines in the spectrum was recorded. While the fire is burning, there are no lines in the spectrum. As coals appear and the radiation power increases, these lines appear and their brightness increases.
What color should the gas burn?
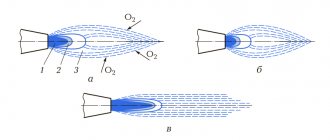
So, in order for the gas to burn as completely as possible and at the same time release the required amount of energy, it is important that there is enough air in the gas mixture. This is achieved by mixing oxygen and burning gas in the burner in the required proportions: for 1 liter of burning gas, 10 liters of air are needed.
Then the frying pan or saucepan will heat up quite quickly, because a lot of heat will be released from the burner. Complete combustion of gas ensures the normal release of heat and some light with the formation of carbon dioxide and water vapor.
This is evidenced by the following signs:
- silent gas combustion;
- blue flame color;
- uniform distribution of flame on all sides of the burner;
- sufficient level of fire;
- ignition without popping;
- there is no combustion stop at the minimum value of the flame power regulator.
If there are any obstacles to the entry of air, gas combustion is incomplete. Oxidation in the presence of insufficient air leads to the formation of carbon monoxide (or carbon monoxide), causing the color to change to yellow or even red.
The more air in the gas mixture, the higher the likelihood that the flame color will be blue and the combustion process will occur correctly
If too much natural gas enters the burner, improper fuel consumption begins, which causes soot to form in the burner. The heat drops, which leads to longer cooking times, and black marks begin to appear on the underside of the cookware after being on the gas stove. All this indicates problems in the operation of the gas burner and the need for urgent cleaning.
We also recommend reading our other channels, where we talked in detail about possible problems with gas burners and ways to solve them:
- The noise of a gas burner.
- Increasing the power of the gas burner.
- The gas burner does not hold a flame.
- The burner on the gas stove does not work.
Discussion of experimental results
Why do we see yellow color, physiology
To correctly explain the results of experiments, we need to understand how our eyes perceive radiation of different wavelengths and how the brain processes this information.
Let me briefly and very, very simply recall well-known facts. We perceive the color yellow for various reasons: in one case, when a narrow spectrum of radiation with a wavelength in the range of 570 nm - 590 nm enters the retina, and in many others, when radiation of a different spectral composition enters the eyes. For example, red and green in the right proportions will be perceived as yellow. On the monitor screen we create just such a yellow color.
That is, our eyes and then the brain create the illusion of color, and therefore, to understand physical and chemical processes, we need to measure the spectrum.
A misconception that is found in many publications that explain the yellow color of the fire - “The color of the fire is caused by sodium radiation”
This experiment shows that the appearance of a double sodium line does not produce any noticeable color change.
Small explanations
Let's compare the emission spectra of the Sun and the flame of a fire.
In the solar spectrum, the maximum is in green, and the power of red and blue is less. Radiation with exactly this spectral characteristic is perceived as white.
In the flame of a fire, carbon atoms form soot particles up to 100 nm in size. These particles produce a continuous spectrum with a maximum of radiation in the infrared region, and the power of visible radiation decreases from red to green and even more to blue. Radiation with such a spectrum is perceived by humans as shades of yellow and orange, depending on the temperature of the flame area. The yellow color of the fire is NOT a coincidence, but more on that below.
Effect of sodium salts
During the combustion process, ash appears which contains salts, including sodium salts. There is very little ash. It begins to rise upward in the flame, and the bright double yellow line of sodium gradually appears in the spectrum. However, its appearance does not noticeably affect the color of the fire, since the eyes already perceive yellow color from the continuous spectrum.
conclusions
The fact that we see a fire yellow does not mean that there is radiation in the narrow spectral range of sodium. Our eyes and brain perceive the continuous spectrum as yellow.
The appearance of an additional bright sodium line has little effect on the perception of the color of the fire, which remains the same yellow. The color change is not noticeable to us, since this color already existed. By the way, if only sodium was responsible for the color of the fire, there would be no shades, since we would see a pure spectral color.
Why does the version that the sodium line gives the fire its yellow color remains popular? Most likely, the coincidence of the color of the sodium line and the black-body spectrum of carbon led to the confusion.
The color of a fire flame comes from brightly glowing carbon particles. The effect of sodium radiation on color is minimal.
Flame color
It is not difficult to guess that the hue of a flame is determined by the chemicals burning in it, if exposure to high temperature releases individual atoms of the combustible substances, coloring the fire. To determine the effect of substances on the color of fire, various experiments were carried out, which we will discuss below.
Since ancient times, alchemists and scientists have tried to find out what substances burn, depending on the color that the flame acquires.
The flames of gas water heaters and stoves, available in all houses and apartments, have a blue tint. When burned, this shade is produced by carbon, carbon monoxide. The yellow-orange color of the flame of a fire that is lit in the forest, or of household matches, is due to the high content of sodium salts in natural wood. This is largely due to the color of the fire truck being red. The flame of a gas stove burner will acquire the same color if you sprinkle it with ordinary table salt. When copper burns, the flame will be green. I think you have noticed that when you wear a ring or chain made of ordinary copper that is not coated with a protective compound for a long time, the skin becomes green. The same thing happens during the combustion process. If the copper content is high, a very bright green light occurs, almost identical to white. This can be seen if you sprinkle copper shavings on a gas burner.
Many experiments have been carried out using an ordinary gas burner and various minerals. In this way their composition was determined. You need to take the mineral with tweezers and place it in the flame. The color that fire takes on can indicate the various impurities present in the element. A green flame and its shades indicate the presence of copper, barium, molybdenum, antimony, and phosphorus. Boron produces a blue-green color. Selenium gives the flame a blue tint. The flame turns red in the presence of strontium, lithium and calcium, and violet in the presence of potassium. The yellow-orange color is produced when sodium burns.
Studies of minerals to determine their composition are carried out using a Bunsen burner. The color of its flame is even and colorless; it does not interfere with the course of the experiment. Bunsen invented the burner in the mid-19th century.
He came up with a method that allows one to determine the composition of a substance by the shade of the flame. Scientists had tried to conduct similar experiments before him, but they did not have a Bunsen burner, the colorless flame of which did not interfere with the progress of the experiment. He placed various elements on a platinum wire into the burner fire, since when this metal is added, the flame does not become colored. At first glance, the method seems good; labor-intensive chemical analysis can be dispensed with. You just need to bring the element to the fire and see what it consists of. But substances in their pure form can be found extremely rarely in nature. They usually contain large quantities of various impurities that change the color of the flame.
Bunsen tried to highlight colors and shades using various methods. For example, using colored glass. Let’s say that if you look through blue glass, you won’t see the yellow color that fire turns when burning the most common sodium salts. Then the lilac or crimson shade of the desired element becomes distinguishable. But even such tricks led to the correct determination of the composition of a complex mineral in very rare cases. This technology could not achieve more.
Nowadays, such a torch is used only for soldering.
A new hypothesis about the influence of fire flames on the adaptive evolution of human color vision
A brief summary of the first part of the publication: (1) in the flame of a fire, two completely different and unrelated mechanisms generate radiation, the colors of which are perceived by humans the same as yellow, (2) sodium radiation changes intensity during the combustion process, (3) the color of the flame in the combustion process does not change and remains yellow.
As you know, we can distinguish many colors. It is stated that there is a million, but even if it were a thousand, the probability of a random color match is 1:1000.
A hypothesis arises logically - this is not accidental. It can be assumed that the fire became a trigger for the evolution of human color vision.
Searches on the Russian-language and English-language Internet do not provide answers. This hypothesis was not only not discussed anywhere, but was not even expressed by anyone. Most likely, this is due to the fact that biologists simply do not know the information about the spectrum of a fire flame that appeared during these measurements.
To make sure that the color of the radiation is really the same, I came up with another elegant experiment
After the flame has finished burning, I scrape the soot from the walls of the fireplace and collect the ash near the coals. I place the soot and ash in two different stainless steel spoons, fill it to the brim with alcohol and set it on fire at the same time. The results are in the pictures below. It turned out beautifully, in my opinion.
I was able to separate the yellow color of the fire flame into two different parts.
What we got. Visually the color matches completely. During the combustion process, the flames of ash and soot look exactly the same. Both the color and burning intensity are the same. But in the pictures you can see a slight difference. In the flame, where soot emits, you can see individual luminous tracks of carbon particles; in ash there are no such things.
Spectral analysis shows that soot emits a continuous spectrum, with little emission at each frequency, and the ash produces two bright sodium lines.
An interesting observation is that the monochrome ash emission flame has different shades of yellow. Most likely, this is due to different radiation powers in different parts of the flame.
Alcohol burns with a dim blue color
Let's return to the discussion of the hypothesis
There are several other facts that make the assumption of the influence of fire on the evolution of color vision more plausible
If the emission color of soot and ash were perceived differently, then (1) the color of the fire would change as it burns and (2) the perceived brightness of the fire would be less.
The location of the perceptual maxima in the three types of retinal cones, 430, 530 and 560 nanometers, is not symmetrical and is shifted towards the sodium line. With this arrangement, the lighting of the fire is much brighter for us (3). How could this happen?
Archaeologists have determined that humans have been using fires for over a million years. During this time, more than 50 thousand generations have changed. It is enough that in each generation the maximum perception in cones changes by 0.001 nm, and in a million years the changes will reach 50 nm (4).
The narrow spectrum of yellow compared to red green and blue further indicates a low probability of coincidence (5).
For millions of years, every day, people spent a significant part of their time around the fire, because the fire was the only alternative source of light, so adaptation was vital (6).
The yellow color of the fire is close in perception to the sunset illumination of the sun, which may be another argument in favor of the hypothesis, since in this way the color of the fire began to be perceived as similar to the color of the sun (7).
Winter sunset on the Ob River
The hypothesis of the influence of fire on the evolution of human color vision may also explain the need for the emergence of trichromatic vision (8).
The popular hypothesis about the evolution of vision for greater convenience in finding fruit among foliage does not explain the need for the emergence of three different cones with maxima at 430 nm, 530 nm and 560 nm. Other primates are dichromats, have color vision and find food easily.
But living with two different light sources could lead to the appearance of trichromatic vision. Let me remind you that the spectral composition of the sun and a fire are very different. The radiation from a fire is more intense in the long wavelength range than in the short wavelength range. And the cones of 530 nm and 560 nm are mainly responsible for color vision near a fire. If there were only one type of cones in this range, and the second type in the violet range, then in the light of a fire a person would have almost monochrome vision. In addition, it is precisely this asymmetrical arrangement of maxima that makes the perception of color in sunlight and firelight very similar, especially for the evening sun.
All the above arguments (1) - (8) are not direct evidence, but they indirectly confirm the hypothesis about the role of the fire in the evolution of human color vision. I consider the main factor to be the extremely low probability of the colors emitted by carbon and sodium particles matching (9).
In conclusion, it should be noted that we can observe two types of evolution - [1] changes in the structure of the eye and [2] adaptations in the brain's processing of information from the eyes. That is, a shift in the maximum perceptions of cones to the sodium lines and the brain’s perception of the same yellow color of radiation from carbon and sodium particles.
A remarkable fact is that due to the fact that during the adaptation process yellow became the most perceived color, for the reasons described above, marketers, road sign developers and game designers began to actively use it.
Causes of flame color changes
Gas equipment is classified as unsafe household appliances. On the one hand, the user should not interfere with its operation and disturb the design of the stove, or try to repair it themselves.
On the other hand, it is important to know the possible signs of breakdowns and respond to them in time so as not to use a hob that has failed.
It is recommended to call gas stove service specialists from time to time to preventively clean the burners and check their functionality.
One of the simplest signs of problems with the stove is a change in the color of the fire. Normally it is blue, but sometimes it can turn orange, red, yellow, and have a pungent and unpleasant odor.
An important condition for normal gas combustion is the required amount of oxygen. There is a certain proportion that must be observed in order for combustion to be as beneficial as possible for the user.
Reasons for changing flame color:
- incomplete combustion of gas;
- incorrect amount of air in the mixture (insufficient or excessive);
- dirty burners;
- inappropriate equipment;
- low quality gas.
There is an opinion that a change in gas color indicates poor quality of the supplied fuel. Allegedly, it is diluted with various substances so that the consumer pays more for the service. In fact, the color of the fire only indicates how correctly the combustion process is carried out.
Thus, a uniform blue color indicates complete combustion of the gas, extracting the maximum amount of heat.
Some stations can dilute blue fuel with other compounds, which, when burned, form soot, soot, and deposits on gas burners
But the supply of low-quality gas cannot be ruled out. This does not affect the color of combustion itself, but poor-quality gas in the future worsens the performance of the stove and leads to the appearance of a yellow flame.
After the stove is in use, black soot may accumulate on it. All this indicates that the flame is smoking. This indicates a gas injection failure. When the burners are operating, there is a lack of gas mixture. This is why gas sometimes burns with a red or yellow flame on the stove.
It is necessary to look for the cause primarily in contamination of the burners, problems with ventilation, etc. The higher the combustion temperature, that is, the more saturated the gas is with oxygen during combustion, the colder the shade of the flame.
Yellow flame color
The air-fuel mixture becomes unusable and changes color for various reasons. The most common is that the holes intended for air intake become clogged. Dust gets into them, which prevents the free passage of air.
This problem is especially relevant in the first years of using gas equipment. This is when it needs to be tested most often. This is due to the fact that after stamping, a small oil film remains on the burner and the ignition group tube for the first time. This leads to dust sticking to its surface, which prevents the normal amount of air from entering. Gas flows in the same amount.
The composition of the mixture changes, and this becomes the reason why the gas burns orange or yellow in the stove, and not the traditional blue or blue.
The gas enters the burner without sufficient oxygen and burns along with soot and dust. It gives that characteristic orange color
It is important to understand that a change in flame color is not the only sign that it is time to clean the stove.
Other indicators are as follows:
- the flame smokes;
- the fire takes on an opaque hue;
- the torch becomes too large;
- the torch becomes glowing.
All this indicates the need to call a technician to clean the burners, their various elements and adjust the operation of the stove so that the air is supplied evenly.
Another common cause of a gas flame turning yellow is that the air control valve is in the wrong position. It may be closed, come off, fall, etc. This causes a lack of air, which leads to loss of heat, soot, yellow flames and other problems. Often this even ends with the need to make urgent repairs to the stove.
The flame burns red
Sometimes the gas can even burn red. The reason for this phenomenon is excess carbon monoxide, which accumulates as a by-product of the combustion of any fuel. If the gas burns with a blue flame, it means that the gas equipment is fully operational and emits a small amount of carbon monoxide.
If the color changes closer to red, there is more and more of this toxic substance. This is quite dangerous, because excessive concentration leads to headaches, nausea, dizziness and other signs of poisoning.
If the gas begins to burn red, and the stove periodically goes out or is difficult to ignite, it’s time to call specialists to clean the gas equipment
The problem with carbon monoxide is that it is odorless and colorless. Therefore, flame color is the only way to recognize an increase in its concentration.
Even small concentrations of this substance (0.01-0.2%) lead to severe symptoms.
The accumulation of carbon monoxide even in small quantities (from 0.01% to 0.2%) can lead to poisoning, manifested by headache, dizziness and nausea
If the gas concentration reaches high levels, it can cause more serious poisoning and even death.
Explanations and instructions
Explanations for those who want to know the details of the experiments, and instructions for those who want to make a similar spectrometer and carry out their own measurements
The design of the device is very simple, but the simplicity was made possible because modern high-tech components were used: a SLR camera, a DVD-R disk, a computer with software for photo processing. I assembled the spectrometer in a rugged case, mounted it on a massive tripod, made replaceable optical slits, and used a mercury lamp with four known mercury emission lines for calibration. Used the database to identify the lines recorded in the experiments. I figured out how to process the data and got a spectrometer resolution of 0.1 nm.
A spectrometer in which the spectrum is recorded by a camera is better suited for experiments on human color perception than classical spectrometers with a uniform power scale. The fact is that manufacturers make a three-color matrix by analogy with human trichromatic vision. We immediately get the desired result.
Making a spectrometer
Fabrication of a diffraction grating
Manufacturing of optical slits
Case manufacturing
Setting up and calibrating the spectrometer
Setting is a choice of matrix sensitivity, lens aperture, exposure, sharpness. All this is done experimentally. The parameters were chosen so that the exposure time when photographing the flame did not exceed 10 seconds.
Calibration was carried out before each series of experiments using the known spectrum of a small-sized mercury fluorescent lamp. The spectrometer was installed on a strong tripod a meter from the flame, a calibration lamp was placed between the device and the fire, and photographs of the lamp's spectrum were taken. Then the lamp was removed, the shutter speed was changed and the spectrum of the flame was photographed.
Processing of measurement results
Processing of measurement results (measurement of wavelengths of the spectrum under study) was carried out as follows: The spectrum of the calibration lamp and the spectrum under study were combined into one frame. Knowing the location of the mercury lines, the required wavelength was determined through measurements and subsequent calculations. The measurements were carried out with an accuracy of one pixel of the camera sensor matrix, which corresponds to 0.1 nm. Three pixels were required to reliably register spectral lines. Half spectral line width 0.3 nm; therefore, the resolution of the spectrometer is no worse than 0.3 nm. Considering that the location of the line centers can be determined with an accuracy of 1 pixel, the wavelength was set with an accuracy of 0.1 nm. Typical homemade spectrometers, information about which can be found on the Internet, have a resolution more than an order of magnitude lower - from one to several nanometers. They are not suitable for such measurements.
I got a little lucky. There are two spectral sources of yellow color in nature: (1) the double line of the emission spectrum of sodium at 589.6 nm and 589.0 nm and (2) the double line of the emission spectrum of mercury at 577.0 nm and 579.1 nm. One of them was in a calibration lamp, the other in the flame of a fire. Between these lines there is only about 10 nm and, accordingly, about 100 pixels. Therefore, I was easily able to measure the wavelength of the sodium line in the fire flame with an accuracy of 0.1 nm.
Read about how to make a high-quality spectrometer and how to conduct experiments correctly in my article “Homemade high-resolution spectrometer”