Test details
A typical test uses a 10 mm (0.39 in) diameter steel ball as an indenter with 3000 kgf (29.42; 6.614 lbf) force. For softer materials, less force is used; For harder materials, the tungsten carbide ball is replaced with a steel ball. The dent is measured and the hardness is calculated as:
BHN = 2 p π D ( D − D 2 − d 2 ) { displaystyle operatorname {BHN} = { frac {2P} { pi D left (D — { sqrt {D^{2}-d^{2))} right)}}}
Where:
BHN = Brinell hardness number (kgf/mm2)
n
= applied load in kilogram-force (kgf)
D
= indenter diameter (mm)
d
= indentation diameter (mm)
Brinell hardness is sometimes expressed in megapascals; The Brinell hardness number is multiplied by the acceleration due to gravity, 9.80665 m/s.2, to convert it to megapascals.
BHN can be converted to ultimate tensile strength (UTS), although the relationship is material dependent and therefore determined empirically. The relationship is based on the Meyer index (n) from Meyer's Law. If the Meyer index is less than 2.2, then the UTS to BHN ratio is 0.36. If the Meyer index is greater than 2.2, then the coefficient increases.[1]
BHN
designated by the most commonly used test standards (ASTM E10-14[2] and ISO 6506–1:2005[3]) as
HBW
(
H
of Hardness,
B
of Brinell, and
W
of tungsten carbide (tungsten) indenter material). In previous standards, HB or HBS were used to refer to measurements made with steel indenters.
HBW is calculated in both standards using SI units as
HBW = 0.102 2 F π D ( D − D 2 − d 2 ) { displaystyle operatorname {HBW} = 0.102 { frac {2F} { pi D left (D — { sqrt {D^{2} -d^{2} }} right)}}}
where:
F
= applied load (newtons)
D
= indenter diameter (mm)
d
= indentation diameter (mm)
Brinell method
When determining the hardness of non-ferrous metals, the Brinell method is used, which consists of pressing a metal ball into the surface of the part, then measuring the diameter of the indentation and converting the values to HB (see Table No. 2). To carry out the described manipulation, you need a special apparatus, but in the absence of it, you can use the same good old Rockwell (“Ball” indenter, load 100 kgf). This way you can control soft metals: aluminum, copper, brass, bronze.
Modern hardness testers have an advanced interface and can connect to a computer and convert hardness values from one method to another automatically. Such equipment is convenient to use and does not require highly qualified operator, but its cost is not always affordable. There are also complaints about ultrasonic hardness testers regarding measurement accuracy. You come to the conclusion that the old, proven over the years, is better than the dubious new at exorbitant prices. If you need to precisely control the hardness after heat treatment, purchase a Soviet-style Rockwell thermal machine; they are made of very high quality and their resource is almost unlimited. This Rockwell will provide precision and breadth of measurements. A cheaper option (but fail-safe) is to determine hardness using a set of calibrated files, although that's a completely different story.
Shared values
When quoting the Brinell hardness number (BHN or more commonly HB), the test conditions used to obtain the number must be specified. (HB is not related to the grade of "HB" hardness of the pencil.) The standard format for defining tests can be seen in the example "HBW 10/3000". "HBW" means that a tungsten carbide ball indenter was used (from the chemical designation for tungsten, or from the Swedish/German name for tungsten "Wolfram"), as opposed to "HBS", which means a hardened steel ball. The number “10” is the diameter of the ball in millimeters. "3000" is the force in kilograms.
Hardness may also be displayed as XXX HB YY. D
2. XXX is the force applied (in kgf) to a material of type YY (5 for aluminum alloys, 10 for copper alloys, 30 for steels).
Thus, the typical hardness of steel can be written: 250 HB 30 D
2. This may be a maximum or a minimum.
The corresponding relationships between scale, indenter and testing force are:
| Hardness symbol | Indenter diameter mm | F/D2 | Test force N/kgf |
| HBW 10/3000 | 10 | 30 | 29420(3000) |
| HBW 10/1500 | 10 | 15 | 14710(1500) |
| HBW 10/1000 | 10 | 10 | 9807(1000) |
Brinell hardness numbers
| Material | Hardness |
| Coniferous wood (eg pine) | 1.6 OBD 10/100 |
| Hardwood | 2.6–7.0 OBD 10/100 |
| News | 5.0 HB (pure lead; alloyed lead can typically range from 5.0 HB to values in excess of 22.0 HB) |
| Pure Aluminum | 15 HB |
| Copper | 35 HB |
| Hardened AW-6060 Aluminum | 75 HB |
| Mild steel | 120 HB |
| 18–8 (304) stainless steel annealed | 200 HB[4] |
| Hardox wear plate | 400-700 HB |
| Hardened tool steel | 600–900 HB (HBW 10/3000) |
| Glass | 1550 HB |
| Rhenium diboride | 4600 HB |
| Note: Standard test conditions unless otherwise stated | |
Brinell hardness table
Brinell hardness is determined by the formula indicated in the table (when the force is expressed in kgf).
When determining Brinell hardness, the indentation diameter d is taken as the arithmetic mean value of the measurement results. Brinell hardness is indicated by a numerical value and the symbol HB, followed by the diameter of the ball and the applied force. Only when the Brinell hardness is determined by a ball with a diameter of 10 mm at a force of 3000 kgf and a holding time of 30 seconds, the designation of the result is only a numerical value and HB, for example 285 HB.
| Table of some (accurate to 0.1) Brinell hardness values , ball diameter 10 mm; d (mm) — diameter of the ball imprint | ||||
| d (mm) | Druckkraft P (kp) | |||
| 3000 | 1000 | 500 | 250 | |
| 2 | 945,76 | 315,25 | 157,63 | 78,81 |
| 2,1 | 856,93 | 285,64 | 142,82 | 71,41 |
| 2,2 | 779,93 | 259,98 | 129,99 | 64,99 |
| 2,3 | 712,75 | 237,58 | 118,79 | 59,40 |
| 2,4 | 653,79 | 217,93 | 108,96 | 54,48 |
| 2,5 | 601,76 | 200,59 | 100,29 | 50,15 |
| 2,6 | 555,61 | 185,20 | 92,60 | 46,30 |
| 2,7 | 514,50 | 171,50 | 85,75 | 42,87 |
| 2,8 | 477,71 | 159,24 | 79,62 | 39,81 |
| 2,9 | 444,65 | 148,22 | 74,11 | 37,05 |
| 3 | 414,85 | 138,28 | 69,14 | 34,57 |
| 3,1 | 387,88 | 129,29 | 64,65 | 32,32 |
| 3,2 | 363,40 | 121,13 | 60,57 | 30,28 |
| 3,3 | 341,10 | 113,70 | 56,85 | 28,43 |
| 3,4 | 320,75 | 106,92 | 53,46 | 26,73 |
| 3,5 | 302,11 | 100,70 | 50,35 | 25,18 |
| 3,6 | 285,00 | 95,00 | 47,50 | 23,75 |
| 3,7 | 269,25 | 89,75 | 44,88 | 22,44 |
| 3,8 | 254,73 | 84,91 | 42,46 | 21,23 |
| 3,9 | 241,31 | 80,44 | 40,22 | 20,11 |
| 4 | 228,88 | 76,29 | 38,15 | 19,07 |
| 4,1 | 217,35 | 72,45 | 36,23 | 18,11 |
| 4,2 | 206,63 | 68,88 | 34,44 | 17,22 |
| 4,3 | 196,65 | 65,55 | 32,77 | 16,39 |
| 4,4 | 187,33 | 62,44 | 31,22 | 15,61 |
| 4,5 | 178,63 | 59,54 | 29,77 | 14,89 |
| 4,6 | 170,49 | 56,83 | 28,41 | 14,21 |
| 4,7 | 162,85 | 54,28 | 27,14 | 13,57 |
| 4,8 | 155,69 | 51,90 | 25,95 | 12,97 |
| 4,9 | 148,96 | 49,65 | 24,83 | 12,41 |
| 5 | 142,63 | 47,54 | 23,77 | 11,89 |
| 5,1 | 136,66 | 45,55 | 22,78 | 11,39 |
| 5,2 | 131,03 | 43,68 | 21,84 | 10,92 |
| 5,3 | 125,71 | 41,90 | 20,95 | 10,48 |
| 5,4 | 120,68 | 40,23 | 20,11 | 10,06 |
| 5,5 | 115,92 | 38,64 | 19,32 | 9,66 |
| 5,6 | 111,41 | 37,14 | 18,57 | 9,28 |
| 5,7 | 107,14 | 35,71 | 17,86 | 8,93 |
| 5,8 | 103,07 | 34,36 | 17,18 | 8,59 |
| 5,9 | 99,21 | 33,07 | 16,54 | 8,27 |
| 6 | 95,54 | 31,85 | 15,92 | 7,96 |
| 6,1 | 92,04 | 30,68 | 15,34 | 7,67 |
| 6,2 | 88,71 | 29,57 | 14,79 | 7,39 |
| 6,3 | 85,53 | 28,51 | 14,26 | 7,13 |
| 6,4 | 82,50 | 27,50 | 13,75 | 6,87 |
| 6,5 | 79,60 | 26,53 | 13,27 | 6,63 |
| 6,6 | 76,82 | 25,61 | 12,80 | 6,40 |
| 6,7 | 74,17 | 24,72 | 12,36 | 6,18 |
| 6,8 | 71,62 | 23,87 | 11,94 | 5,97 |
| 6,9 | 69,19 | 23,06 | 11,53 | 5,77 |
| 7 | 66,85 | 22,28 | 11,14 | 5,57 |
Note on the Brinell hardness table: the values highlighted in gray are calculated and cannot be used in practice.
Advantages
| This section may be confusing or unclear to readers . |
It can be used to test heterogeneous materials (materials whose properties are not constant throughout the sample). Brinell makes it possible to test using different forces and different indenters. The testing process is non-destructive.
Importance for plastics recycling
Brinell hardness, although Rockwell is the more common method, has a wide range of applications in the production of plastics and elastomers. Mainly, numerical values of hardness are used to assess the quality of manufacturing of polymer equipment and technological equipment.
In the field of polymer engineering, the hardness of components and assemblies cannot be neglected. Despite the apparent softness and pliability of the molten polymer, it can quite easily damage and disable equipment that does not have sufficient quality of the metal from which it is made.
Fig.2. Checking the quality of equipment parts
In particular, when accepting molds for plastic injection molding from low-cost manufacturers from China, it is an extremely common practice to evaluate the hardness of the molding parts. This process is carried out using a hand-held hardness tester directly on the surface of the tooling, slightly to the side of the forming area. The most demanding customers bring their own equipment to the mold acceptance site.
As for polymers and elastomers themselves, as stated earlier, the most common method in the world (with the exception of Japan and some other countries) are hardness testers operating on the Shore and A and D scales. The Brinell method can only be suitable for particularly heavily filled polymers , but it is too inaccurate for standard high-volume plastics.
Standards
- International (ISO) and European (CEN) Standard "EN ISO 6506-1: 2005: Metallic materials - Brinell hardness test - Part 1: test method".
- "EN ISO 6506-2: 2005: Metallic materials - Brinell hardness test - Part 2: Verification and calibration of the testing machine."
- "EN ISO 6506-3: 2005: Metallic materials - Brinell hardness test - Part 3: Calibration of reference samples."
- "EN ISO 6506-4: 2005: Metallic materials - Brinell hardness test - Part 4: Table of hardness values."
- "ASTM E10-14: Standard Test Method for Brinell Hardness of Metallic Materials."
Chemical side of hardness
An interesting phenomenon is the difference in hardness of allotropic modifications of diamond. The fact is that stone consists of carbon atoms. Apart from this substance there should be nothing in it. Of course, in nature there are impurities that spoil the structure and affect the hardness. But this does not affect the performance of most specimens.
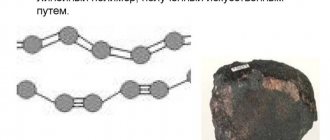
Crystal lattice of diamond and graphite
Not only are diamonds entirely composed of carbon, the substance has many allotropic modifications, but they are no longer so hard. So, from the element the following materials are obtained:
- graphite;
- carbon nanotubes;
- coal and soot;
- fullerenes;
- lonsdaleite.
Among all modifications, graphite has been well studied; it is this material, together with talc, that has a hardness on the Mohs scale equal to one. In fact, this difference in hardness is explained by the crystalline structure of the atomic lattice.
Carbon atoms in diamond are arranged in the form of tetrahedrons - these are figures with four faces, which at the corners contain carbon atoms connected to each other by covalent sigma bonds. Sigma bonds are the strongest in chemistry, and a material like diamond consists entirely of them. In graphite, in turn, planar bonds are also covalent sigma structures, but spatial ones are covalent pi bonds, which are less strong and unstable to breaking. Pi bonds involve the presence of free electrons, so graphite has little electrical conductivity.
Fullerenes are medium in hardness, since their lattice at the corners contains not atoms, but carbon molecules. The bonds between atoms in a molecule are very strong, but covalent bonds between molecules can be broken. It is the complexity of building connections that explains the difficulty in creating an artificial diamond. It is possible to destroy connections between each other, but building them and obtaining a solid mineral from graphite is extremely difficult. This requires special conditions for pressure and temperature.
A diamond's strength, or hardness, can only be tested by applying gradual pressure. If the pressure on the material increases sharply, then this will be considered a mechanical impact, in other words, an impact. A gradual increase in the indicator will reveal either the plasticity or hardness of the substance.
Scientists have come up with a material that is 58% harder than this mineral; they called it lonsdaleite. Moreover, the composition of the material is identical - carbon atoms. The substance can withstand pressure 55 GPa more than diamond, but obtaining such a crystalline structure and synthesizing lonsdaleite is a very difficult and costly job. Therefore, the use of lonsdaleite is limited.