Butane, production, properties, chemical reactions.
Butane, C4H10, is an organic substance of the alkanes class. It is found in nature in natural gas extracted from gas and gas condensate fields, and in associated petroleum gas. It is also formed during cracking of petroleum products.
Butane, formula, gas, characteristics
Physical properties of butane
Chemical properties of butane
Butane production
Chemical reactions - equations for butane production
Applications and uses of butane
General formula and isomerism
One of the representatives of alkanes is isobutane. The formula of this class has the form SpH2n+2. The molecule has a linear structure in which each carbon atom is in the Sp3 hybrid state. The presence of single (simple) bonds in the molecule explains the similarity in chemical properties of branched alkanes with substances that have a straight skeleton.
What types of isomerism does isobutane have? The formula of this substance is C4H10 or (CH3)3CH. Normal butane has the same molecular appearance. Consequently, isobutane is characterized by isomerism of the carbon skeleton (structural type). No interclass isomers were identified for representatives of saturated hydrocarbons.
Butane, formula, gas, characteristics:
Butane is an organic substance of the alkanes class, consisting of four carbon atoms and ten hydrogen atoms. The name comes from the root “but-” (the French name for butyric acid is acide butyrique) and the suffix “-an” (which means it belongs to alkanes).
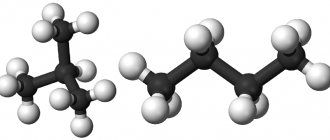
The chemical formula of butane is C4H10. It has two isomers n-butane and isobutane. In chemistry, the name "butane" is used primarily to refer to n-butane. The mixture of n-butane and its isomer isobutane has the same name.
Rational formula of n-butane CH3-CH2-CH2-CH3, isobutane CH(CH3)3.
Structure of the n-butane molecule:
Structure of the isobutane molecule:
Butane is a colorless, tasteless gas with a specific characteristic odor.
It is found in nature in natural gas extracted from gas and gas condensate fields, and in associated petroleum gas. To separate natural and associated petroleum gas, they are purified and gas is separated.
It is also formed during cracking of petroleum products, incl. shale oil.
Also found in shale gas and LPG (liquefied natural gas).
Fire and explosion hazard.
Slightly soluble in water and other polar solvents. But it dissolves in some non-polar organic substances (methanol, acetone, benzene, carbon tetrachloride, diethyl ether and others).
It is low toxic, but has a harmful effect on humans - on the nervous system (poisoning, vomiting, death is possible), has narcotic properties, can cause suffocation and cardiac arrhythmia, and causes dysfunction of the pulmonary-respiratory system. Hazard class four.
Properties and scope of isobutane
Definition 1
Isobutane is a hydrocarbon belonging to the class of alkanes and is an isomer of normal butane.
Under normal conditions, isobutane is a gaseous substance. If there is an increase in the number of hydrocarbon atoms in the molecule, then a transition occurs to a liquid state of aggregation, and then to a solid. It has a low degree of solubility in water, but is highly soluble in organic compounds.
The general chemical formula of isobutane is as follows - $(CH_3)_3CH$. Thus, isobutane is not able to undergo addition reactions. The class of alkanes is characterized by radical substitution. Since isobutane has a mobile carbon atom, the substitution occurs much faster than butane, which has a normal structure.
Are you an expert in this subject area? We invite you to become the author of the Directory Working Conditions
Isobutane is able to interact with halogens (bromine, chromium) under the influence of light. This property is used to obtain monohydric alcohols. Isobutane is also in demand as a refrigerant. Isobutane was once thought to have a negative impact on the ozone layer, destroying its integrity. The high octane number of isobutane makes it possible to use it as a special additive to gasoline. It can be used as a fuel for lighters, as well as in the perfume industry and medicine. Isobutane is also used:
- In the production of foamed polymer materials based on polystyrene, polyethylene, etc.
- In the manufacture of polyurethane foam.
- In the production of automotive enamels, repellents, aerosol paints.
Physical properties of butane:
| Parameter name: | Meaning: |
| Color | no color |
| Smell | specific characteristic odor |
| Taste | no taste |
| Physical state (at 20 °C and atmospheric pressure 1 atm.) | gas |
| Density (state of matter – liquid, at 0 °C), kg/m3 | 601,2 |
| Density (state of matter – gas, at 0 °C), kg/m3 | 2,672 |
| Melting point of n -butane , °C | -138,4 |
| Melting point of isobutane , °C | -159,6 |
| Boiling point of n -butane , °C | -0,5 |
| Boiling point of isobutane , °C | -11,7 |
| Auto-ignition temperature, °C | 372 |
| Critical temperature*, °C | 152,01 |
| Critical pressure, MPa | 3,797 |
| Critical specific volume, m3/kg | 228 |
| Explosive concentrations of gas-air mixture, % volume | from 1.4 to 9.3 |
| Specific heat of combustion , MJ/kg | 45,8 |
| Molar mass, g/mol | 58,12 |
* at temperatures above the critical temperature, the gas cannot be condensed at any pressure.
Liquefied petroleum gas (LPG), liquefied petroleum gas (LPG)
Liquefied petroleum gas (LPG) is one of the types of alternative fuel.
LPG is a mixture of propane, normal butane, isobutane, propylene, ethane, ethylene and other hydrocarbons.
Methods for obtaining LPG:
- as a product of oil refining at oil refineries (refineries),
- in the production of oil and natural gas.
The use of a mixture of these gases as fuel is due to a number of physical and chemical properties:
- high boiling points at atmospheric pressure - allow you to store a liquefied propane-butane mixture in the operating temperature range: - 40°C - + 45°C at relatively low pressure (up to 1.6 MPa);
- LPG does not lose or change its properties over time and does not erode.
- octane number of LPG is more favorable in comparison with gasoline and diesel fuel and varies in the range of 90 -110, depending on the ratio of propane and butane in the mixture.
- The energy efficiency of LPG is lower than that of traditional fuels due to the low energy per unit. volume. This increases combustion consumption by 10-20% compared to gasoline fuel, but is compensated by 2 times lower price.
- LPG burns more efficiently and safely even in a cold engine, even when the engine is cold, burns relatively cleanly, without smoke and ash, that is, it is more environmentally friendly.
Compared to diesel fuel:
- 90% less particulate matter,
– 90% less nitrogen oxides,
— 60% less carbon dioxide CO2,
— LPG does not pollute the soil because it does not dissolve in water.
Each of the gas components has a certain boiling point, so the pressure of the LPG vapor phase depends on both the temperature and its component composition.
The component composition of liquefied hydrocarbon gas is regulated by GOST 20448-90 “LIQUEFIED HYDROCARBON GASES FOR MUNICIPAL CONSUMPTION”.
The standard provides for 3 brands of gas:
- PT (technical propane),
- SPBT (a mixture of technical propane and butane),
- BT (technical butane).
The content of propane, butane and other impurities in LPG affects many of its properties, because it significantly affects the octane number and vapor density of the fuel.
Octane number (ON) is an indicator of the fuel’s resistance to detonation:
- grows due to an increase in the content of saturated hydrocarbons (propane, n-butane, isobutane, etc.),
- unsaturated hydrocarbons polymerize, which contributes to the formation of sediment - carbon deposits in the tank, in the fuel system and combustion chamber.
Vapor pressure (mixture volatility) is very important in low ambient temperatures. Keeping it at the appropriate level allows the LPG to exit the tank. Both components of the mixture are gaseous and low-boiling.
Propane boils at atmospheric pressure already at -42 ° C, butane, under the same temperature conditions at -0.5 ° C, therefore, in winter, the propane content in the fuel gas is increased to increase the gas vapor pressure.
In summer the mixture ratio is about 40% propane and 60% butane, while in winter the ratio is the opposite: 60/40.
Propane is more expensive than butane, so the “winter” mixture is also more expensive than the “summer” mixture.
Gas stations must monitor the composition of the mixture and not cheat by replacing the winter mixture with a summer one.
Unlike gas filling stations, CNG filling stations use compressed network natural gas from gas pipelines.
LPG production technologies:
- directly from crude oil: during production, associated petroleum gas (APG) is released,
- When stabilized in tanks, ethane, propane, butane and pentane are released.
Chemical properties of butane:
Butane is difficult to react chemically. Under normal conditions, it does not react with concentrated acids, molten and concentrated alkalis, alkali metals, halogens (except fluorine), potassium permanganate and potassium dichromate in an acidic environment.
The chemical properties of butane are similar to those of other representatives of a number of alkanes. Therefore, it is characterized by the following chemical reactions:
- 1. catalytic dehydrogenation of butane:
CH3-CH2-CH2-CH3 → CH2=CH-CH2-CH3 + H2 (kat = Pt, Ni, Al2O3, Cr2O3, increased to).
- 2. butane halogenation:
CH3-CH2-CH2-CH3 + Br2 → CH3-CHBr-CH2-CH3 + HBr (hv or increased to);
CH3-CH2-CH2-CH3 + I2 → CH3-CHI-CH2-CH3 + HI (hv or increased to).
The reaction is chain in nature. A molecule of bromine or iodine under the influence of light breaks down into radicals, then they attack butane molecules, tearing off a hydrogen atom from them, as a result of which free butyl CH3-CH·-CH3 is formed, which collides with bromine (iodine) molecules, destroying them and forming new ones iodine or bromine radicals:
Br2 → Br++Br(hv); – initiation of the halogenation reaction;
CH3-CH2-CH2-CH3 + Br· → CH3-CH·-CH2-CH3 + HBr; – growth of the halogenation reaction chain;
CH3-CH·-CH2-CH3 + Br → CH3-CHBr-CH2-CH3 + Br·;
CH3-CH·-CH2-CH3 + Br· → CH3-CHBr-CH2-CH3; – break of the halogenation reaction chain.
Halogenation is one of the substitution reactions. The least hydrogenated carbon atom is halogenated first (tertiary atom, then secondary, primary atoms halogenate last). The halogenation of butane occurs in stages - no more than one hydrogen atom is replaced in one stage.
CH3-CH2-CH2-CH3 + Br2 → CH3-CHBr-CH2-CH3 + HBr (hv or increased to);
CH3-CHBr-CH2-CH3 + Br2 → CH3-CBr2-CH2-CH3 + HBr (hv or increased to);
etc.
Halogenation will continue to occur until all hydrogen atoms have been replaced.
- 3. butane nitration:
See ethane nitration.
- 4. oxidation (combustion) of butane:
With excess oxygen:
2C4H10 + 13O2 → 8CO2 + 10H2O.
When there is a lack of oxygen, instead of carbon dioxide (CO2), carbon monoxide (CO) is produced; with an even smaller amount of oxygen, finely dispersed carbon soot is released (in various forms, including in the form of graphene, fullerene, etc.) or a mixture of them.
- 5. butane sulfochlorination:
C4H10 + SO2 + Cl2 → C4H9-SO2Cl + … (hv).
- 6. butane sulfoxidation:
2C4H10 + 2SO2 + O2 → 2C4H9-SO2OH (increased to).
Obtaining butane. Chemical reactions - equations for butane production:
Since butane is contained in sufficient quantities in natural gas, associated petroleum gas and is released during the cracking of petroleum products, it is not produced artificially. It is isolated during purification and separation from natural gas, APG and oil during distillation.
Butane in laboratory conditions is obtained as a result of the following chemical reactions:
- 1. hydrogenation of unsaturated hydrocarbons, for example, butene:
CH3-CH2-CH=CH2 + H2 → CH3-CH2-CH2-CH3 (kat = Ni, Pt or Pd, increased to).
- 2. reduction of haloalkanes:
C4H9I + HI → C4H10 + I2 (increased to);
C4H9Br + H2 → C4H10 + HBr.
- 3. interaction of haloalkanes with alkali metal, for example, sodium (Wurtz reaction):
2C2H5Br + 2Na → CH3-CH2-CH2-CH3 + 2NaBr;
2C2H5Cl + 2Na → CH3-CH2-CH2-CH3 + 2NaCl.
The essence of this reaction is that two haloalkane molecules bond into one, reacting with an alkali metal.
- 4. alkaline melting of salts of monobasic organic acids:
C4H9-COOH + NaOH → C4H10 + Na2CO3 (increased to);
C4H9-COONa + NaOH → C4H10 + NaHCO3.
Receipt
For the laboratory production of isobutane, the isomerization reaction of a hydrocarbon with a straight-chain structure is used. To do this, the corresponding hydrocarbon is exposed to active metals acting as catalysts. Isobutane is also produced in the laboratory in this way. The structural formula of this compound confirms the branching of its structure.