| Isobutane | |
| Isobutane3.png | |
| Are common | |
| Systematic name | methylpropane |
| Traditional names | isobutane |
| Chem. formula | (CH3)3CH |
| Physical properties | |
| State | gas |
| Molar mass | 58.12 g/mol |
| Thermal properties | |
| T. float. | -159.6 °C |
| T. kip. | -11.73 °C |
| Classification | |
| Reg. CAS number | 75-28-5 |
| SMILES | [chemapps.stolaf.edu/jmol/jmol.php?model=CC%28C%29C CC(C)C] |
| Data given is based on standard conditions (25 °C, 100 kPa) unless otherwise stated. | |
Isobutane
(methylpropane, 2-methylpropane) (CH3)3CH is a hydrocarbon of the alkane class, an isomer of normal butane (n-butane).
What is freon R600a or isobutane
Refrigerant R600a (isobutane, freon 600a) – natural gas. It has zero potential for destruction of the Earth's ozone layer and minimal impact on the greenhouse effect. It is an alternative to the obsolete R12 freon.
The chemical formula of freon r-600a is similar to butane, natural gas. Butane is the refrigerant of R600. The prefix in the name iso means that, despite the same chemical composition, gas molecules have different structures. This is designated by the letter "a" in the refrigerant classification.
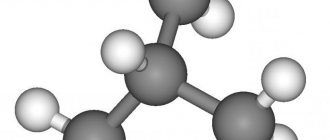
On the left is an isobutane molecule, on the right is butane. Dark balls correspond to carbon atoms, light balls correspond to hydrogen atoms.
Nomenclature[edit]
The traditional name isobutane was still retained in the 1993 IUPAC guidelines[21], but is no longer recommended as per the 2013 guidelines.[1] Because the longest continuous chain in isobutane contains only three carbon atoms, the preferred IUPAC name is 2-methylpropane , but the locant (2-) is usually omitted in general nomenclature as redundant; C2 is the only position in the propane chain where a methyl substituent can be present without changing the main chain and forming the constitutional isomer n-
butane
Background
Due to its good characteristics, R600a (HC 600a) refrigerant has been used in refrigerators since the beginning of the 20th century. After the advent of R12 refrigerant, isobutane was no longer used due to the fact that a lot of it was required, 200-400 grams in one household refrigerator.
In large volumes, freon r600a was a fire hazard in such quantities. In the event of a leak, it could ignite from an open flame or an electric spark and lead to a fire. Isobutane was forgotten for decades.
In the 20th century, refrigeration technology was constantly improved. By the 1990s, a household refrigerator system needed no more than 150 grams of isobutane to operate. In addition, after the signing of the Montreal Protocol, the R12 refrigerant began to be phased out. Freon R600a has become the main refrigerant for household refrigeration and freezing equipment.
Quality assurance
We carefully select not only the manufacturer, but also each batch of goods. As a matter of principle, we do not work with goods of dubious quality at a lower price, with which the markets of both China and Russia are flooded. That is why the largest world-famous enterprises trust us.
› ‹
- 1
- 2
- 3
- 4
- 5
We supply Freon R-600a throughout Russia: Moscow, St. Petersburg, Novosibirsk, Yekaterinburg, Nizhny Novgorod, Samara, Kazan, Omsk, Chelyabinsk, Rostov-on-Don, Ufa, Volgograd, Perm, Krasnoyarsk, Voronezh, Tver, Kirov , Yaroslavl, Novgorod, Murmansk, Saratov, Krasnodar, Togliatti, Irkutsk, Naberezhnye Chelny, Barnaul, Nizhnevartovsk, Tomsk, Kaliningrad and any other city of the Russian Federation and the Customs Union.
Production of R600a, isobutane (n-butane)
R600a refrigerant is produced by rearranging butane (R600) molecules. This process is called isomerization. The number of hydrogen and carbon atoms in the molecule remains the same, but their geometric structure changes.
Butane isomerization occurs in a butameric unit using a catalyst made of platinum or other rare earth metals. During the process, only part of the gas is converted to n-butane. The resulting mixture is passed through a deisobutanization distillation column, in which R600a is separated from R600.
Honeywell butameric installation.
Homologous series of butane
All alkanes are substances that are similar in physical and chemical properties and differ by one or more –CH2– groups from each other. Such substances are called homologues , and a series of substances that are homologues is called a homologous series .
The very first representative of the homologous series of alkanes is methane CH4. , or Н–СH2–H.
The homologous series can be continued by sequentially adding a –CH2– group to the hydrocarbon chain of the alkane.
| Alkane name | Alkane formula |
| Methane | CH4 |
| Ethane | C2H6 |
| Propane | C3H8 |
| Butane | C4H10 |
| Pentane | C5H12 |
| Hexane | C6H14 |
| Heptane | C7H16 |
| Octane | C8H18 |
| Nonan | C9H20 |
| Dean | C10H22 |
The general formula of the homologous series of alkanes is CnH2n+2.
The first four members of the homologous series of alkanes are gases, C5–C17 are liquids, and starting from C18 are solids.
Physical properties of freon R600a, isobutane
R600-a is a colorless, odorless gas. It has a low boiling point, which allows it to be used in climate control and cooling technology. It does not contain chlorine and fluorine, unlike gases such as R12 and R134a.
Refrigerant gas R600a is flammable. When storing, transporting and using it, safety precautions must be observed. Under high pressure, its ignition temperature decreases. 5.45 kg of isobutane is pumped into a regular freon cylinder, 2.5 times less than r134a (tetrafluoroethane) or r12.
The raw materials for the production of isobutane are natural gases extracted from the bowels of the Earth. It can be obtained from products of oil refining, cracking, and other hydrocarbon conversion processes.
Due to its characteristics, r600a refrigerant is used as a component of refrigerants R406a, R413A, R414A, R414B, R422A, R422B, R422C, R422D, R422E, R424A, R428A, R429A, R430A, R434A R439A, R436A, R436B, R 441A, R443A, R461A, R510A.
Interesting fact
Isobutane is often used as a filler in aerosol cans, lighters and refill cans.
Gaseous
Propane belongs to the organic substances of the alkanes class. Propane is found in natural gas and can be formed during the cracking of petroleum products. Propane is considered one of the most poisonous gases.
Physical properties
Propane is a colorless gas that is slightly soluble in water. The boiling point of propane is 42.1C. When in contact with air, propane forms an explosive mixture (at a vapor concentration of 2 to 9.5%). At a pressure of 760 mmHg, the combustion temperature of propane can be about 466 °C.
Chemical properties
The chemical properties of propane are similar to most of the properties of a number of alkanes. These properties include: chlorination, dehydrogenation, and so on.
Propane Applications
Propane is widely used as a fuel for various needs. It is an important component of liquefied hydrocarbon gases. Propane is used for the production of solvents and in the food industry (as a propellant, additive E944).
Refrigerant
A mixture of isobutane (R-600a) and pure propane (R-290a) does not harm the ozone layer and has a low greenhouse potential (GWP). Therefore, this mixture is widely used as a refrigerant. This mixture has replaced obsolete refrigerants in refrigeration and air conditioning units.
Butane (C4H10) - like propane, belongs to the class of alkanes. This is an organic compound that is very toxic and causes poisoning to the human body if inhaled. In chemistry, butane is usually called a mixture of n-butane and its isomer isobutane CH(CH3)3. The name butane consists of two parts, the root “but-”, which in English means butyric acid, and the ending “-an”, which indicates that this substance is an alkane.
Isomerism
Butane has two isomers:
melting point, °C
boiling point, °C
Physical properties
Butane is a colorless and flammable gas. At normal pressure and temperatures below 0 °C it liquefies easily. With increased pressure and normal temperature, it is a highly volatile liquid. The solubility of butane in water is 6.1 mg per 100 milliliters of water. Butane at a pressure of 10 atmospheres and a temperature of 100 °C can form an azeotropic compound with water.
Finding and receiving
Butane is found in oil and gas condensate (its share is approximately 12%). Butane is also produced by hydrocatalytic or catalytic cracking of petroleum fractions. In laboratory conditions, butane is obtained by the Wurtz reaction:
Applications and reactions
Free radical chlorination produces a mixture of 2-chlorobutane and 1-chlorine. Combustion in air produces water and carbon dioxide. Butane is widely used as a mixture with propane in lighters and gas cylinders. In them it is in a liquefied state and has a certain odor due to the presence of odorants in the mixture. There are “summer” and “winter” mixtures, which have different compositions. The heat of combustion of one kilogram of butane is approximately 45 MJ (12.72 kWh).
When there is a lack of oxygen, soot or carbon monoxide or both is formed.
DuPont has patented a method for producing maleic anhydride by catalytic oxidation from n-butane
n-Butane is a good raw material for the production of butene, 1,3-butadiene, which are important components of high octane gasoline. Pure butane is used as a refrigerant in refrigeration and air conditioning units. Butane is better than freon due to its environmental friendliness and safety for the environment, but is less productive than freon refrigerants. Butane is registered as food additive E943a in the food industry, and isobutane is registered as additive E943b, propellant. These substances are used in deodorants.
In the food industry, butane is registered as a food additive E943a , and isobutane - E943b , as a propellant, for example in deodorants.
The effect of butane on the human body
Human inhalation of butane can cause heart failure and death from asphyxiation. Contact with liquid butane or a jet of butane gas causes cooling to minus twenty degrees, which is very dangerous for humans.
Advantages of R600a refrigerant
- To refill a household refrigerator, 25 to 150 grams of gas are required. This is less than in equipment operating on other types of freons.
- Isobutane is cheaper than other refrigerants.
- Freon R600a provides high cooling capacity. It runs the most economical household refrigerators of classes A+, A++, A+++.
- The production of isobutane does not involve complex chemical processes. O is available and cheap.
- R600-a refrigerant is chemically stable and does not react with metals, rubbers and plastics.
- Isobutane refrigeration uses cheap mineral oil rather than expensive synthetic oil.
- Due to the smaller amount of gas in the refrigerator system, the noise level is reduced.
Receipt
For the laboratory production of isobutane, the isomerization reaction of a hydrocarbon with a straight-chain structure is used. To do this, the corresponding hydrocarbon is exposed to active metals acting as catalysts. Isobutane is also produced in the laboratory in this way. The structural formula of this compound confirms the branching of its structure.
Features of isobutane
Compared to other refrigerants, R600a freon has higher cooling capacity. For full operation of the equipment, less of it is required. Therefore, the cost of refrigeration circuit components is reduced. This leads to cheaper household appliances.
When replacing r12 freon with r600, no major equipment modifications are required. It is only necessary to change the filter drier and extend the capillary tube. The cost of such modification is minimal. Considering the difference in price of freons r12 and r600a, transporting a refrigerator using isobutane is quite justified.
A small spark is enough to ignite isobutane. The gas ignites if its content in the air remains from 14 to 85 cubic meters. cm per liter, or from 31 to 205 grams. At a different concentration it will not ignite. But there is not much of it in refrigerators, so the likelihood of a fire is minimal, although it does exist.
If there is a small leak, the gas will burn in the immediate vicinity of the leak. If it comes out all at once and ignites, it will instantly flare up and burn. In this case, the fire will not be able to spread to other objects.
Many sites steal this quote from each other without thinking about its meaninglessness:
Modern designs of household and commercial refrigeration equipment contain an acceptable concentration of R600. The dose of filling household refrigerators with isobutane is so small that it practically cannot lead to a fire: if the refrigerant completely leaks from the unit, its concentration in a kitchen with a volume of 20 cubic meters will be tens of times lower than the flammability threshold.
Probably, the authors of the articles did not study physics at all in high school. Isobutane tends to accumulate at the bottom of the room. Its concentration will vary depending on altitude. If the room is not ventilated and a leak has occurred, at a certain height from the floor the ratio of isobutane to air will be within the explosive range .
The claim that r600a refrigerant is dangerous is false. Isobutane is not toxic or poisonous. Poisoning can only occur at very high concentrations in the air. If there is a leak from a household refrigerator, it is absolutely safe.
Due to its safety and chemical stability, R600a freon does not need to be disposed of. When repairing the system or converting it to another refrigerant, the remaining old gas is simply released into the atmosphere.
Interesting fact
Refrigeration equipment designed to use isobutane as a refrigerant has special requirements. It is designed in such a way that if there is a leak in a place where gas accumulates, it cannot ignite. There are no electrical or electronic components that can ignite the gas.
Links[edit]
- ^ a b Organic Chemistry Nomenclature: IUPAC Recommendations and Preferred Names 2013 (Blue Book)
. Cambridge: Royal Society of Chemistry. 2014. p. 652. DOI: 10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. The names "isobutane", "isopentane" and "neopentane" are no longer recommended. - "Solubility in water". PubChem
. National Center for Biotechnology Information. Retrieved April 6, 2022. - "CDC - NIOSH Pocket Guide to Chemical Hazards - Isobutane". CDC - NIOSH Pocket Guide to Chemical Hazards
. CDC. Retrieved December 28, 2022. - ^ a b
Entry in the GESTIS substance database of the Institute for Occupational Safety and Health - ^ a b c
NIOSH Pocket Guide to Chemical Hazards. "#0350" . National Institute of Occupational Safety and Health (NIOSH). - "Patent Watch, July 31, 2006" . Archived from the original on March 11, 2007. Retrieved August 8, 2006.
- Bipin V. Vora; Joseph A. Kokal; Paul T. Barger; Robert J. Schmidt; James A. Johnson (2003). "Alkylation". Kirk-Othmer Encyclopedia of Chemical Technology
. DOI: 10.1002/0471238961.0112112508011313.a01.pub2. ISBN 0471238961. - Kenneth S. Whiteley. "Polyethylene". Ullman Encyclopedia of Industrial Chemistry
. Weinheim: Wiley-VCH. DOI: 10.1002/14356007.a21_487.pub2. - Jump up
↑ Rietveld, Will (2005-02-08). "Frequently asked questions about lightweight canister stoves and fuels". Camping light. Retrieved July 26, 2014. - "European Commission on the Modernization of Refrigerants for Stationary Applications" (PDF). Archived from the original (PDF) on 08/05/2009. Retrieved October 29, 2010.
- Page - March 15, 2010 (2010-03-15). "GreenFreeze". Greenpeace. Retrieved January 2, 2013.
- "EPA Hydrocarbon Refrigerants Frequently Asked Questions". EPA.gov. Retrieved October 29, 2010.
- "Policy Statement Compilation on Hydrocarbons and Refrigerants, October 2006." (PDF). Archived from the original (PDF) on 08/08/2014. Retrieved August 1, 2014.
- "MACS Bulletin: Hydrocarbon Refrigerant Use in Vehicles" (PDF). Archived from the original (PDF) on January 05, 2011. Retrieved October 29, 2010.
- "Society of Automotive Engineers Bulletin on Hydrocarbon Refrigerants". sae.org. 2005-04-27. Archived from the original on 2005-05-05. Retrieved October 29, 2010.
- "Saskatchewan Labor Bulletin on Hydrocarbon Refrigerants in Vehicles". labor.gov.sk.ca. 2010-06-29. Archived from the original on 2009-07-01. Retrieved October 29, 2010.
- VASA on refrigerant legality and feasibility Archived January 13, 2009, at the Wayback Machine
- "Queensland (Australia) Government Hydrocarbon Refrigerant Alert" (PDF). energy.qld.gov.au. Archived from the original (PDF) on December 17, 2008. Retrieved October 29, 2010.
- "Parliamentary Record of New South Wales (Australia), 16 October 1997". parliament.nsw.gov.au. 1997-10-16. Archived from the original on July 1, 2009. Retrieved October 29, 2010.
- "New South Wales (Australia) parliamentary report, 29 June 2000". parliament.nsw.gov.au. Archived from the original on May 22, 2005. Retrieved October 29, 2010.
- Panico, R. and Powell, W. H., eds. (1994). IUPAC Manual of Nomenclature for Organic Compounds 1993
. Oxford: Blackwell Science. ISBN 0-632-03488-2. https://www.acdlabs.com/iupac/nomenclature/93/r93_679.htm
Refrigerant r600a, technical characteristics
| Characteristics of R600a | Meaning |
| Molecular (molar) mass, g/mol | 58,12 |
| Molecular formula | С4Н10 |
| Boiling point at 0.1 MPa,ºС | -11,73 |
| Freezing temperature at 0.1 MPa,ºС | -159,6 |
| Flash point, °C | -85 |
| Density at 25ºС, g/cc | 0,551 |
| Evaporation pressure at 25°C, MPa | 0,498 |
| Critical temperature, °C | 135 |
| Critical pressure, MPa | 3,65 |
| Critical density, g/cc | 0,221 |
| Latent heat of vaporization, kJ/kg | 366,5 |
| Explosive limits of volume fraction in air, % | 1,8 — 8,4 |
| Cooling efficiency of mixture with air, J/g | 150,7 |
| Solubility in oil | Full |
| Volume of saturated liquid, l/kg | 0,844 |
| Ozone Depletion Potential, ODP | 0 |
| Greenhouse potential, GWP | 0 |
| Annual greenhouse potential, HGWP | 0,001 |
| CAS number | 75-28-5 |
The following impurities are possible in isobutane in negligible quantities: methane, ethane, water, propane, propylene, N-butane, pentene, butene-1, isobutylene, isopentane, N-pentane, carbon dioxide, sulfur, nitrogen, oxygen.
Decarboxylation of carboxylic acid salts (Dumas reaction)
The Dumas reaction is the interaction of salts of carboxylic acids with alkalis during fusion.
R–COONa + NaOH → R–H + Na 2 CO 3
Decarboxylation is the removal (elimination) of a carbon dioxide molecule from a carboxyl group (-COOH) or an organic acid or carboxylate group (-COOMe) of a salt of an organic acid.
When sodium pentanoate reacts with sodium hydroxide during fusion, butane and sodium carbonate are formed:
CH3–CH2–CH2– CH2 –COONa + NaOH → CH3–CH2 – CH2 – CH3 + Na 2 CO 3
Working pressure suction r600a in refrigerator
| t, °С | Abs., bar | Rel., bar | t, °С | Abs., bar | Rel., bar |
| -40 | 0,29 | -0,71 | +15 | 2,62 | 1,62 |
| -35 | 0,38 | -0,62 | +20 | 3,02 | 2,02 |
| -30 | 0,47 | -0,53 | +25 | 3,54 | 2,54 |
| -25 | 0,62 | -0,38 | +30 | 4,05 | 3,05 |
| -20 | 0,73 | -0,27 | +35 | 4,69 | 3,69 |
| -15 | 0,82 | -0,18 | +40 | 5,32 | 4,32 |
| -10 | 1,09 | 0,09 | +45 | 6,09 | 5,09 |
| -5 | 1,33 | 0,33 | +50 | 6,86 | 5,86 |
| 0 | 1,57 | 0,57 | +55 | 7,79 | 6,79 |
| +5 | 1,89 | 0,89 | +60 | 8,72 | 7,72 |
| +10 | 2,21 | 1,21 | +70 | 10,91 | 9,91 |
Requirements for the purity of freon refrigerant R600a according to AHRI 700
| Testing | Result |
| Appearance | Visually transparent |
| Particulate matter | Visually clean |
| Isobutane content | more than 99.5% |
| Sum of all impurities | up to 0.5% |
| Water content | less than 10 ppm |
| Acidity | less than 1 ppm |
| Freon gas content in the container | up to 0.01% |
* ppm, parts per million (English) - parts per million.
Results of the study of freon R600a
Impact on human health
In case of contact with eyes, frostbite may occur due to rapid evaporation of the substance. Contact with skin due to rapid evaporation may cause frostbite. It manifests itself in a change in skin color from white to gray, and the formation of blisters.
Isobutane is heavier than air and can cause suffocation by reducing the amount of air available to breathe. This can lead to loss of consciousness and death. Preliminary effects can be:
- Dizziness;
- Disorientation;
- Vomiting or nausea;
- Sleepy state;
- Impaired cardiovascular activity.
Type of substance
Food additive E 943b is included in the group of propellants.
Isobutane is an isomer of normal butane (n-butane E 943a). The substance belongs to the category of saturated hydrocarbon gases of organic nature.
The product is contained in natural gas condensate and is part of petroleum gases. It is obtained during the processing of feedstock or as a result of butane isomerization. The reaction catalyst is sulfated metal oxides (usually zirconium).