Tarnish colors are colors that become visible on a smooth metal or mineral surface due to the appearance of a thin oxide film or light interference in it. Often their appearance is associated with thermal exposure.
Metal tarnish colors spread due to the redistribution of light intensity in thin films on the reflection structure. As the film thickness develops, conditions for the extinction of rays from a certain wavelength appear.
At what temperature does metal turn red?
Already in ancient times, people mined and smelted copper.
This metal was widely used in everyday life and served as a material for the manufacture of various objects. They learned to make bronze about 3 thousand years ago. This alloy was used to make good weapons. The popularity of bronze quickly spread, as the metal was distinguished by its beautiful appearance and durability. Jewelry, hunting and labor tools, and dishes were made from it. Thanks to the low melting point of copper, people quickly mastered its production. The metal received its Latin name Cuprum from the name of the island of Cyprus, where they learned to mine it in the third millennium BC. e. In the periodic system, Cu received number 29, and is located in the 11th group of the fourth period.
In the earth's crust, the element is in 23rd place in distribution and is most often found in the form of sulfide ores. The most common are copper luster and pyrite. Today, copper is extracted from ore in several ways, but any technology requires a step-by-step approach to achieve results.
- At the dawn of the development of civilization, people were already obtaining and using copper and its alloys.
- At that time, it was not sulfide ore that was mined, but malachite ore, which did not require pre-roasting.
- A mixture of ore and coals was placed in a clay vessel, which was lowered into a small pit.
- The mixture was ignited, and carbon monoxide helped the malachite to be restored to the state of free Cu.
- There is native copper in nature, and the richest deposits are in Chile.
- Copper sulfides often form in medium-temperature geothermal veins.
- Often the deposits are in the form of sedimentary rocks.
- Copper sandstones and shales are found in Kazakhstan and the Chita region.
Physical properties
The metal is ductile and in the open air it becomes covered with an oxide film in a short time. Thanks to this film, copper has its yellowish-red tint; in the lumen of the film, the color can be greenish-blue. In terms of thermal and electrical conductivity, Cuprum is in second place after silver.
- Density - 8.94×103 kg/m3.
- Specific heat capacity at T=20 ° C - 390 J/kg x K.
- Electrical specific at 20−100 ° C - 1.78×10−8 Ohm/m.
- Boiling point - 2595 ° C.
- Specific electrical conductivity at 20 ° C is 55.5−58 MS/m.
At what temperature does copper melt?
Melting occurs when a metal changes from a solid state to a liquid state. Each element has its own melting point. Much depends on the impurities in the metal. The normal melting point of copper is 1083 ° C. When tin is added, the temperature drops to 930-1140 ° C. The melting point here depends on the tin content of the alloy. In an alloy of cuprum and zinc, melting occurs at 900-1050 ° C.
When any metal is heated, its crystal lattice is destroyed. As it heats up, the melting point increases, but then levels off once a certain temperature limit is reached. At this moment the metal melts. It melts completely and the temperature rises again.
When the metal cools, the temperature decreases, at a certain point it remains at the same level until the metal hardens completely. After complete hardening, the temperature drops again.
This is demonstrated by the phase diagram, which shows the temperature process from the beginning of melting to solidification. When heated, heated copper at 2560 ° C begins to boil. Boiling is similar to the boiling of liquid substances, when gas is released and bubbles appear on the surface.
At the moment of boiling at the highest possible temperatures, the release of carbon formed during oxidation begins.
Melting at home
Due to its low melting point, ancient people could melt cuprum over a fire and use the metal to make various products.
To melt copper at home you will need:
- charcoal;
- crucible and special tongs for it;
- muffle furnace;
- household vacuum cleaner;
- bugle;
- steel hook;
- melting mold.
The process proceeds in stages, the metal is placed in a crucible and then placed in a muffle furnace. The desired temperature is set, and the process is monitored through a glass window. During the process, an oxide film will appear in the container with Cu, which needs to be removed - open the window and move it to the side with a steel hook.
Is it possible to increase the hardness of metals and their alloys?
Technologies for imparting greater hardness to metals and alloys have been improved over many centuries. Modern equipment makes it possible to carry out heat treatment in such a way as to significantly improve the properties of products even from inexpensive materials.
Hardening of steel and alloys
Hardening (martensitic transformation) is the main method of imparting greater hardness to steels. In this process, the product is heated to such a temperature that the iron changes its crystal lattice and can be additionally saturated with carbon. After holding for a certain time, the steel is cooled.
This must be done at high speed to prevent the formation of intermediate forms of iron. As a result of rapid transformation, a solid solution supersaturated with carbon with a distorted crystal structure is obtained. Both of these factors are responsible for its high hardness (up to HRC 65) and brittleness.
When hardening, most carbon and tool steels are heated to a temperature of 800 to 900C, but high-speed steels P9 and P18 are heated at 1200-1300C.
Microstructure of high-speed steel R6M5: a) cast state; b) after forging and annealing; c) after hardening; d) after vacation. ×500.
Quenching modes
The heated product is lowered into a cooling medium, where it remains until it cools completely. This is the simplest hardening method, but it can only be used for steels with a low carbon content (up to 0.8%) or for parts of simple shape. These limitations are associated with thermal stresses that arise during rapid cooling - parts of complex shapes can warp or even crack.
With this method of hardening, the product is cooled to 250-300C in a saline solution for 2-3 minutes to relieve thermal stress, and then cooling is completed in air. This helps prevent cracks or warping of parts. The disadvantage of this method is the relatively low cooling rate, so it is used for small (up to 10 mm in diameter) parts made of carbon or larger ones made of alloy steels, for which the hardening rate is not so critical.
It begins with rapid cooling in water and ends with slow cooling in oil. Typically, such hardening is used for products made of tool steels. The main difficulty lies in calculating the cooling time in the first environment.
Surface hardening (laser, high frequency currents)
Used for parts that must be hard on the surface, but have a viscous core, for example, gear teeth. During surface hardening, the outer layer of the metal is heated to supercritical values, and then cooled either during the heat removal process (with laser hardening) or by liquid circulating in a special inductor circuit (with high-frequency current hardening)
Temperature table for quenching and tempering steels
| No. | steel grade | Hardness (HRCe) | Temperature hardening, degrees C | Temperature holidays, degrees C | Temperature zak. HDTV, deg.C | Temperature cement., deg. C | Temperature annealing, degrees C | Temper. Wednesday | Note |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1 | Steel 20 | 57…63 | 790…820 | 160…200 | 920…950 | Water | |||
| 2 | Steel 35 | 30…34 | 830…840 | 490…510 | Water | ||||
| 33…35 | 450…500 | ||||||||
| 42…48 | 180…200 | 860…880 | |||||||
| 3 | Steel 45 | 20…25 | 820…840 | 550…600 | Water | ||||
| 20…28 | 550…580 | ||||||||
| 24…28 | 500…550 | ||||||||
| 30…34 | 490…520 | ||||||||
| 42…51 | 180…220 | Sech. up to 40 mm | |||||||
| 49…57 | 200…220 | 840…880 |
Hardening of steel parts
Hardening gives the steel part greater hardness and wear resistance.
To do this, the part is heated to a certain temperature, held for some time so that the entire volume of the material warms up, and then quickly cooled in oil (structural and tool steels) or in water (carbon steels).
Typically, parts made from structural steels are heated to 880–900°C (light red incandescent color), those from instrumental steels are heated to 750–760°C (dark cherry red color), and those from stainless steel are heated to 1050–1100°C ( color dark yellow).
The parts are heated slowly at first (to about 500°C), and then quickly. This is necessary to ensure that internal stresses do not arise in the part, which can lead to cracks and deformation of the material.
In repair practice, they mainly use cooling in one medium (oil or water), leaving the part in it until it cools completely. However, this cooling method is unsuitable for parts with complex shapes, in which large internal stresses arise during such cooling.
Parts of complex shape are first cooled in water to 300–400°C, and then quickly transferred to oil, where they are left until completely cooled. The residence time of the part in water is determined at the rate of 1 s for every 5–6 mm of the part’s cross-section. In each individual case, this time is selected empirically depending on the shape and mass of the part.
The quality of hardening largely depends on the amount of coolant
It is important that during the cooling process of the part, the temperature of the coolant remains almost unchanged, and for this its mass must be 30–50 times greater than the mass of the part being hardened. In addition, before immersing a hot part, the liquid must be thoroughly mixed to equalize its temperature throughout the entire volume.
During the cooling process, a layer of gases forms around the part, which impedes heat exchange between the part and the coolant. For more intense cooling, the part must be constantly moved in the liquid in all directions.
Colors of Heat are... What are Colors of Heat?
For the film, see White Heat (film)
Colors of Heat
- these are the colors of the glow of metal heated to a high temperature. The spectrum of thermal radiation depends on temperature, so by observing the colors of incandescence you can quickly, although without high accuracy, determine the temperature of the metal, which is often used in heat treatment and forging. Moreover, before the invention of non-contact thermometers, this was the only way to judge the temperature of a metal. Abbreviated names for heat colors ("red heat", "white heat") are often used by metallurgists instead of indicating temperature.
Dependence of incandescence color on temperature
The table lists the heat colors characteristic of steel.
| Temperature, °C | Heat color |
| 550 | dark brown |
| 630 | brown-red |
| 680 | Dark red |
| 740 | dark cherry |
| 770 | cherry |
| 800 | bright or light cherry |
| 850 | bright or light red |
| 900 | bright red |
| 950 | yellow-red |
| 1000 | yellow |
| 1100 | bright or light yellow |
| 1200 | yellow-white |
| 1300 | white |
Phraseologism
The expression “bring to a white heat” also has a well-known figurative meaning: “to make you angry,” “to drive you crazy,” “to infuriate you.”
Selection of lighting equipment by Tc value
The functional approach to determining the required light temperature differs from design and special tasks. In the first case, we take into account the requirements of technical standards and experience accumulated in medicine, manufacturing, design, and architecture. In the second, we rely on aesthetic preferences and the logic of decorative solutions. In the third, we fulfill the design requirements.
Light temperature in functional lighting
There are 2 main types of functional lighting - general and local. Depending on the purpose of the room/zone/object/, it is recommended to use equipment with Tc values in the range of 2400… 7000 K.
| Recommended color temperature of artificial lighting, K | ||
| Space | General knowledge | Local development |
| Living rooms | 2800… 4200 | 2400… 4200 |
| Bedrooms | 2400… 3200 | 2400… 3500 |
| Children's | 2800… 3200 | 2800… 3500 |
| Common areas | 3200… 5500 | 3500… 5500 |
| Kitchens in apartments | 2800… 3200 | 3500… 5500 |
| Classes of educational institutions | 3200… 4500 | |
| Offices | 4000… 6500 | 4000… 6500 |
| Recreation areas | 2200… 3200 | 2200… 3000 |
| Warehouses | 3200… 5500 | 3200… 7000 |
| Workshops | 4000… 7000 | 4000… 7000 |
| Printing houses | 6500 | 6500 |
| Advertising agencies | 4000… 5500 | 4000… 6500 |
| Highways | 3500… 5000 | |
| Parks, boulevards | 5000… 7000 | 5000… 7000 |
The color temperature of LED lamps can correspond to any range indicated in the table. Therefore, the actual choice between LED and another type of IC will depend not on Tc, but on other technical or economic parameters.
Rice. 7. Edison lamps are one of the few areas where LEDs are still losing
Light temperature and design challenges
By selecting lamps of a certain spectral characteristic, the designer can:
- emphasize the advantages and soften the shortcomings of the room - for example, poisonous green walls will become delicately light green if you fill them with an orange (2200 K) stream; vulgar flashy red will be softened by backlighting with ordinary yellow (3200 K); the room will increase in size if you emphasize the verticals and horizontals with blue (7000 K) soffits;
- create a special emotional atmosphere - Edison lamps (2000 K) will help emphasize the intimacy and comfort of a bar, cafe, lounge area; cold bluish lighting will add romanticism and pathos to the hall of ancient sculpture in the museum; UV lamps (7000… 9000 K) in a nightclub will emphasize the graphic nature of the dancers’ poses and give the figures an alien mystique;
- effectively convey the color characteristics of the product on the store window by placing - meat - under IC 2800... 3500 K; fish - under metal halide or LED lamps with a color temperature of 4000... 6500 K; jewelry - under lighting 5500... 6500 K; furniture - under warm lamps, and curtains and textiles - under cool white ones.
Tc special ICs
To perform certain technological tasks, it is possible to use ICs with a narrow range of light waves. Water disinfection installations and air disinfection lamps contain bactericidal lamps with a light temperature of 12,000 K or more. Sources of 10,000...15,000 K are also used for curing composite adhesives and structural composites in engineering and dentistry.
Rice. 8. Disinfection of subway cars with bactericidal UV lamps
Sodium, metal halide and narrow spectrum LED sources are used in crop production. The required values of their light temperature depend on the stage of plant growth.
Where do they appear?
A change in color occurs during oxidation, which occurs due to heating of the metal. During the heating process, color tones change in the same sequence, but at different speeds (depending on the increase in temperature and heating duration).
Due to the fact that the pattern of color changes is known, in the past blacksmiths relied on this fact to know how the temperature changes. With the development of technology, a pyrometer appeared.
Color tones for steel
If it makes sense to describe the pattern of changes in the color of tarnish for carbon steel depending on the degree of heating:
- straw - after 220,
- brown - up to 240–250,
- raspberry - 250–270,
- violet-blue - from 300,
- gray - from 350.
If alloy steel is used, color changes must be expected with a further increase in heating temperature.
In nature
In addition to steel, in wild conditions there are minerals on which a thin layer of oxide film forms. The color of the tarnish in this case can be golden, red, blue, or greenish. The red color of tarnish in natural minerals can be caused by the large number of chromophores contained in its composition. The violet-blue color may arise from the concentration of transition metal ions.
Due to the shade of the oxide film, the natural color of the mineral is not visible. If glass or a coin lies under a layer of soil for a long time, a film forms on its surface, which can change the color of the surface of the object.
Rainbow shades occur due to the presence of a fatty film. Also, the color of the steel surface changes due to water and minerals that have dried on it.
The color changes according to a certain pattern, however, this is not an accurate indicator of temperature. When working on metal, you need to use a pyrometer.
Origin
The color tone of tarnish belongs to interference colors. Visually, they change under different lighting and viewing angles. Also, the physical and chemical properties of the metal affect the color change of the material.
Physics of the process
After the steel surface begins to heat up, tarnish appears, which quickly changes color, ranging from yellow to gray. Depending on the temperature (more than 500 degrees), the first tones of heat appear, noticeable only in complete darkness.
If the temperature exceeds 650 degrees, the metal becomes red-hot. At high temperatures, the color of the oxide film can change from cherry to white (at 1100–1200 degrees). With further heating, the white will only become brighter, but will not change. The heating color of a metal surface is not an accurate indicator of temperature.
Optical effects
The color tone depends on the thickness of the oxide film. When it increases, colors with a short wavelength range are extinguished. As the temperature increases, the film thickness increases. Thus, certain shades of oxides begin to disappear. First purple disappears, then yellow, then green and red disappear. This is the so-called interference of light.
Color tone due to heat
Hardening and tempering in artisanal conditions – Blacksmithing
The topic was created for those who are taking their first steps in heat treatment, I’d like to warn you right away that I’m not far from a thermal guru, but I understand a little, please don’t ask difficult questions and don’t put me in a dead end. So first, some fairly general remarks - heating temperature is controlled by color heat, controlled “by eye” in dim daylight, with a certain skill you can distinguish a temperature difference of about 50 degrees, heat colors start at about 550 degrees (but this is noticeable only in the twilight) A good guide in determining the heating temperature of a part is also the magnetic properties steel, namely at a temperature of 768 degrees (and above) the steel does not magnetize, cooling below this point the magnetic properties return, so by heating the part and periodically testing it with a magnet for “adhesion”, you can understand that the temperature has reached 768 degrees, remember the color of heat that is at the same time I was already more confident in navigating the colors of heat, but you can continue to use a magnet, especially if the lighting is either too bright or, on the contrary, too dark and the colors are not perceived quite properly. This is roughly what the colors look like and what they are called. Why do they look like this? because it’s not exactly like in the picture (I haven’t found the exact colors on the Internet), here are a couple of real photos with heat colors and temperature. But again, on my monitor they look at the indicated temperature, yours may look a little different. It will time (and interest in the topic) I will continue.
Modified on 16.10.2013 15:44 by sanek66
Method for measuring temperatures by tarnish and heat colors
The method of measuring temperatures by the colors of tarnish and heat has been successfully used since ancient times by metallurgists, blacksmiths, thermal experts, as well as representatives of other professions, including machine operators. To measure temperature using this method, tables are used that contain patterns of tarnish and heat colors with a description of their shades and an indication of the temperatures that lead to the appearance of each of them.
Craftsmen and specialists who have regular practice usually do not use tables. Because they know all the color shades and temperature values associated with their manifestations by heart. When there is no constant practice in this area, it is probably not worth relying on memory, especially color memory. By visual comparison from one table or another, a template is selected whose color is more similar to the color of the controlled area of the object
I would like to draw your attention to the fact that when comparing the colors of a template and an object, you should not expect them to completely match until they are identical.
The similarity of their color shades is enough. And then we can assume that the temperature of a uniformly heated object is in the range of values indicated on the color template.
Often two adjacent colors appear on the surface of an object at once. It is not difficult to guess that the temperature of this object is between the average temperatures indicated on both templates. Compared to instrumental measurements, the accuracy of this method is, of course, lower. And yet, in many applications, for example, when performing not particularly important hardening or tempering, the accuracy of the color method is quite sufficient. As for cutting, when the distance of the cutting edge is controlled by the colors of tarnish on the moving chips, and at different points, there is probably no replacement for this old method. Enough tables with the colors of tarnish and heat have been published in the literature and on the Internet. Their interpretations differ in form and content, unfortunately, too. Unlike most of them, the colors used in this template video tutorial are computer matched to real heat colors and the tarnish colors of carbon steels. The names of color shades indicated on the templates are conditional. And their exact identification is possible using the so-called html color code indicated below.
Using this code entered into the search, the color of any of their templates can be easily found on the Internet. Ready-made tables with color templates for downloading to a mobile device or for printing can be downloaded from the project website. Possible causes of errors when measuring temperatures It must be taken into account that color perception is affected by the general illumination of the room, as well as its color, which can be natural, white or yellowish, coming from incandescent lamps. This applies to cases when trying to evaluate colors relying on memory. When measuring temperatures by tarnish colors, you need to understand that they reflect the temperature on the controlled surface. And this does not always correspond to the temperature of the entire mass of the heated object. If the task is to heat an object to a certain temperature, controlled by the color of the tarnish, it must be heated not through one point or surface, but evenly, from all sides. The uniformity of heating is also controlled by the colors of the heat. The same glow color at different points in any area of the object indicates its uniform heating. And vice versa. The scale that peels off from the hot base cools and heats up faster than the base mass, which distorts the actual color of the surface. This must be taken into account.
Source
Types of heat treatment
Heat treatment (heat treatment) of steel, non-ferrous metals is the process of changing the structure of steel, non-ferrous metals, alloys during heating and subsequent cooling at a certain speed. Heat treatment (heat treatment) leads to significant changes in the properties of steel, non-ferrous metals, and alloys. The chemical composition of the metal does not change.
Annealing is a thermal treatment (heat treatment) of a metal that involves heating the metal and then slowly cooling it. This heat treatment (i.e. annealing) comes in different types (the type of annealing depends on the heating temperature and the cooling rate of the metal).
Hardening
Hardening is a heat treatment (heat treatment) of steel and alloys, based on the recrystallization of steel (alloys) when heated to a temperature above critical; After sufficient exposure to the critical temperature to complete the heat treatment, rapid cooling follows. Hardened steel (alloy) has a nonequilibrium structure, so another type of heat treatment is applicable - tempering.
Vacation
Tempering is a heat treatment (heat treatment) of steel and alloys, carried out after hardening to reduce or relieve residual stresses in steel and alloys, increasing toughness, reducing the hardness and brittleness of the metal.
Normalization
Normalization is a heat treatment (heat treatment) similar to annealing. The differences between these heat treatments (normalization and annealing) are that during normalization the steel is cooled in air (when annealing, it is cooled in a furnace).
Heating the workpiece is a critical operation. The quality of the product and labor productivity depend on the correctness of its implementation. You need to know that during the heating process the metal changes its structure, properties and characteristics of the surface layer and as a result of the interaction of the metal with atmospheric air, scale is formed on the surface; the thickness of the scale layer depends on the temperature and duration of heating, the chemical composition of the metal. Steels oxidize most intensively when heated above 900°C; when heated to 1000°C, oxidation increases 2 times, and at 1200°C - 5 times.
Chrome-nickel steels are called heat-resistant because they practically do not oxidize.
Alloy steels form a dense, but not thick layer of scale, which protects the metal from further oxidation and does not crack during forging.
Hardening knife steel at home
For simple carbon steels, even in artisanal conditions, satisfactory hardening can be done, the main thing is to arm yourself with the right knowledge.
Used tools, springs and files can be used as sources; Make sure there is no rust on them. A workpiece made from brand new melted metal is, of course, better, since parts that have served for a long time have a quality called fatigue, which reduces their strength. Although for high-quality materials it is enough to carry out annealing, which consists of heating the steel, holding it at a certain temperature and then slowly cooling it with a furnace or in sand at a speed of two to three degrees per minute. As a result of annealing, a stable structure is formed, free from residual stresses.
For both annealing and heating the part for hardening, you can use a homemade forge made from a pit lined with bricks, a blowtorch and a pipe. Ideally, of course, use a muffle furnace.
It’s easy to check at home whether the hardening has reached the required degree: you can run a file over the hardened product - if the hardening is not complete, the file will simply stick to the knife. Overheating can be checked in artisanal conditions by a strong blow of the workpiece against a hard object - a stone or a rail: the overheated blade shatters into pieces with such a blow.
Directory: Steel marking
Steel marking is done with indelible paint, regardless of the steel group and degree of deoxidation. By agreement of the parties, paint marking is not performed. Letters and numbers for steel: Grades of carbon steel of ordinary quality are designated by the letters St and number (StO, St1, StZ, etc.). High-quality carbon steels are marked with two-digit numbers showing the average carbon content in hundredths of a percent: 05; 08; 10; 25; 40, etc. The letter G in the steel grade indicates a high Mn content (14G; 18G, etc.). Automatic steels are marked with the letter A (A12, A30, etc.). Carbon tool steels are marked with the letter U (U8; U10; U12, etc. Here the numbers indicate the steel content in tenths of a percent).
The first digits of the grade indicate the average carbon content in steel (in hundredths of a percent for structural steels and tenths of a percent for tool and stainless steels). Then the letter indicates the alloying element. The numbers following the letter indicate its average content in whole units. When the alloying element content is less than 1.5%, numbers are not placed after the corresponding letter. The letter A at the end of the brand designation indicates that the steel is high quality. Letter Ш – especially high quality. Ordinary quality steel St0; VSt0, BSt0 – Red and green St1, VSt1kp – Yellow and black St2, VSt2kp – Yellow StZ, VStZkp, VStZ, BStZkp, BStZ – Red St4, VSt4kp, VSt4, BSt4kp, BSt4 – Black St5, VSt5 – Green St6 – Blue
Quality carbon steel 08, 10, 15, 20 – White 25, 30, 35, 40 – White and yellow 45, 50, 55, 60 – White and brown
Alloy structural steel Chrome - Green and yellow Chrome-molybdenum - Green and purple Chrome-vanadium - Green and black Manganese - Brown and blue Chrome-manganese - Blue and black Chrome-silicon - Blue and red Chrome-silicon-manganese - Red and purple Nickel-molybdenum - Yellow and purple Chrome-nickel - Yellow and black Chrome ony-nickel-molybdenum – Purple and black Chrome-aluminium – Aluminum
Corrosion-resistant steel Chrome - Aluminum and black Chrome-nickel - Aluminum and red Chrome-titanium - Aluminum and yellow Chrome-nickel-silicon - Aluminum and green Chrome-nickel-titanium - Aluminum and blue Chrome-nickel-niobium - Aluminum and white Chrome-manganese-nickel - Aluminum and brown Chrome-nickel-molybdenum Ethane – Aluminum and Violet
High speed steel P18 – Bronze and red P9 – Bronze
Hard sintered alloys VK2 – Black with a white stripe VKZ-M – Black with an orange stripe VK4 – Orange VK6 – Blue VK6-M – Blue with a white stripe VK6-V – Purple VK8 – Red VK8-V – Red with a blue stripe VK10 – Red with white stripe VK15 – White T15K6 – Green T30K4 – Blue
At what temperature does steel turn red?
When hardening many tools, such as hammers, hammers, cutters and others, it is required that only the working part be hardened, and the tool itself remains raw, unhardened. In this case, the tool is heated slightly above the working end to the required temperature, after which only the working part is lowered into water. Having taken the tool out of the water, quickly clean its working part with sandpaper or rubbing it on the ground. The heat remaining in the uncooled part will raise the temperature of the cooled end and the desired tarnish color will appear on it, after which the tool is finally cooled.
Table 7 Table for determining the heating temperature by tarnish colors
| Tarnish color | Temperature, degrees WITH | A tool to be released |
| Pale yellow | 210 | – |
| Light yellow | 220 | Turning and planing tools for machining cast iron and steel |
| Yellow | 230 | Same |
| Dark yellow | 240 | Coins for coining by casting |
| Brown | 255 | – |
| Brown-red | 265 | Dies, taps, drills, cutters for processing copper, brass, bronze |
| Violet | 285 | Chisels for steel processing |
| Dark blue | 300 | Coining coins for sheet copper, brass and silver |
| Light blue | 325 | – |
| Grey | 330 | – |
The formation of scale on the surface of the product leads to waste of the metal and deformation. This reduces thermal conductivity and, therefore, reduces the rate of heating of the product in the furnace and complicates mechanical processing. Scale is removed either mechanically or chemically (etching).
Tempering colors of metals: what are they and how do they occur?
For people whose field of activity is far from metalworking, the phrase “tarnished color” not only says nothing, but even poses a kind of mystery. Indeed, what intriguing and unusual does this concept hide?
In fact, everything is much more prosaic and ordinary, and almost every adult has seen traces of discoloration. Let's look into this issue.
What are tarnish colors?
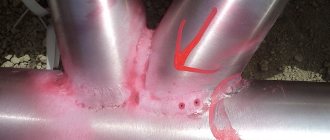
These are the colors of the rainbow spectrum, formed on the smooth or polished surface of materials.
The tarnish of metals and alloys with a gray color is most pronounced, although it also quite often forms on the surface of copper, bronze and brass, as well as on crystals of minerals, for example, chalcopyrite, bornite, bismuth.
And this terminology is most often used by people whose profession is in one way or another connected with various types of processing of steels and other metals and alloys:
- welding and surfacing;
- heat treatment;
- turning and milling.
The colors of tarnish allow them to roughly determine the temperature of the impact on the metal even before instrumental control, because the peculiarity of this physical phenomenon is partial irreversibility. That is, if tarnish colors have already appeared on the metal, then either they cannot be removed at all, or removal will require the use of mechanical, chemical or thermal treatment.
One way or another, in most cases it is possible to get rid of them, but an experienced specialist, as a rule, can accurately identify traces of such additional processing.
Nevertheless, the appearance of rainbow colors on metal products is not always a sign of a defect; this feature of metals is used in jewelry, in the manufacture of firearms and bladed weapons, and in other areas.
How do tarnish colors appear?
Rainbow iridescence or individual tarnish colors arise due to the redistribution of light intensity as a result of the superposition of several light waves.
Similar interference of light occurs in microscopic transparent films of oxides formed on reflective surfaces, and oxidation of metals occurs as a result of exposure to certain chemical reagents or thermal energy.
The lower the heating temperature, the thinner the oxide film, respectively, the more spectral components in the reflected rays and the lighter the color of the tarnish. Conversely, the more the metal heats up, the thicker the oxide film and the darker its hue due to the absorption of long light waves.
The presence of rainbow overflow is not always, but quite often, a defect or indicates a violation of metalworking technology.
For example, during turning, the output of dark blue chips indicates that the cutting speed was clearly exceeded or that the cutting fluid (coolant) was not used. But tarnished colors when hardening metal products are considered the norm.
Annealing and hardening of duralumin
Annealing
Duralumin is produced to reduce its hardness. The part or workpiece is heated to approximately 360°C, as during hardening, held for some time, and then cooled in air. The hardness of annealed duralumin is half that of hardened duralumin.
The approximate heating temperature of a duralumin part can be determined as follows. At a temperature of 350–360°C, a wooden splinter, which is passed along the hot surface of the part, becomes charred and leaves a dark mark. A fairly accurate temperature of the part can be determined using a small (about the size of a match head) piece of copper foil, which is placed on its surface. At a temperature of 400°C, a small greenish flame appears above the foil.
Annealed duralumin has low hardness; it can be stamped and bent twice without fear of cracks.
Hardening
. Duralumin can be hardened. When hardening, parts made of this metal are heated to 360–400°C, held for some time, then immersed in water at room temperature and left there until completely cooled. Immediately after this, duralumin becomes soft and flexible, easily bent and forged. It acquires increased hardness after three to four days. Its hardness (and at the same time fragility) increases so much that it cannot withstand bending at a small angle.
Duralumin acquires its highest strength after aging. Aging at room temperature is called natural, and at elevated temperatures - artificial. The strength and hardness of freshly quenched duralumin, left at room temperature, increases over time, reaching its highest level after five to seven days. This process is called duralumin aging.
What do you need to know about tarnish colors for stainless steel?
When welding stainless steel alloy, rainbow weld colors can occur over a wider range of heat (from 300 to 700 degrees). The color may vary from blue to light yellow depending on the degree of heating. But in the case of corrosion-resistant steels, this is a sign indicating that the chromium oxide layer, which performs the function of protecting the metal product from rust, has been damaged. Therefore, no matter what color the weld seam appears in this case, it should be remembered that corrosion may subsequently occur.
In addition, you may be interested in our separate article devoted to the features of servicing welding equipment.
What caused
A color change upon heating indicates that an oxide film several molecules thick is formed on the surface of the heated material. The color varies depending on its density and thickness. The larger the size and density of the oxides, the more significantly the color tone will differ from the original one.
Some people believe that the color tone of the tarnish can accurately indicate the degree of heating, but this is an erroneous statement. The appearance of different shades is influenced by time, heating rate, the content of various impurities, and the nature of lighting. If we talk about alloy steels, then they need to be heated up more.
A little physics
Radiation emanating from a physical body can consist of 3 streams of photons:
- reflected - the smoother the surface, the more reflective it is. Different substances reflect selectively (rays of some colors are absorbed and others are reflected). Selective reflection explains the rationale for using dyes;
- refracted - a characteristic of transparent and translucent media through which rays pass, deflecting at a certain angle;
- emitted - depends on the intensity of heating of the substance.
The characteristics of radiation are determined only by the thermal energy of the body, regardless of the type of substance. Each temperature of an object corresponds to streams of photons with a certain wavelength, perceived by the eye (and interpreted by the brain) of a person as having a fixed color. Therefore, color temperature is the color of emitted light, expressed in Kelvin temperature scale values.
A degree in this scale is denoted by the letter K. In dimension it is equal to a degree Celsius. The only difference is the zero mark. Zero according to Kelvin is the same “absolute zero” at which the elementary particles of matter are motionless and the body does not emit anything. 0 K corresponds to -273.15 °C.
Color temperature is equal to the real measure of heating only in so-called “absolute black bodies” (ABB). These are abstract objects that serve as models in theoretical physics, which emit but do not reflect or refract anything.
Rice. 1. A completely black body emits light in the visible spectrum solely as a result of heating
A number of substances behave like blackbody in certain temperature ranges. For example, for molten iron heated to 2000 K, Tc = 2000 K. But for a gas flame, the difference is very significant: Tc = 9000 K with real T = 1200 K. This happens because the flame not only emits, but refracts and reflects the “alien” light passing through it and its own emitted light. Another reason for the discrepancy is spectral shift, but consideration of this concept is beyond the scope of the topic.
Rice. 2. Molten steel emits light as a black body, and the Tc of a gas flame (9000 K) is much higher than its actual temperature (1200 K)
The labeling of lamps that we use as light sources (LS) must include the color temperature value in Kelvin. In some cases, it is necessary to convert this characteristic into light wavelength or vice versa. The relationship between two quantities is expressed by an approximate formula:
λm Tc ≈ 3000 µm K.
Lamp color rendering index CRI
Methods for soldering LED strips
The comfort of staying indoors and productivity are affected not only by the brightness of the light, but also by its shade. No less important is the correspondence of the perceived color to the real one. The numerical designation of this parameter is called the index or color rendering coefficient. It is designated Ra or CRI, from the English. color rendering index.
The reference is daylight. Its CRI is 100. Lighting manufacturers do not strive to achieve this quality. Lamps with a coefficient of more than 80 do not tire the eyes, and with Ra more than 90 they are subjectively no different from the reference ones.
Difference in color display at different CRI
When determining Ra, eight reference colors (DIN 6169) are compared using the International Commission on Illumination (CIE) method. In this case, a distortion of the color of the samples under the studied lighting from the color under the reference lighting is noted. Lamps with Tc up to 5000K are compared with a reference luminaire that produces a black body spectrum, and for luminaires with higher temperatures the reference is daylight.
The average deviation is subtracted from 100. The result is the color rendering index CRI.
Groups that contain the ranges of the light source
Nowadays, light sources are divided into three groups:
- Warm glow, white. This is the heat of the glow ranging from 2700 to 3200 Kelvin. It is considered advisable to use such lighting in living rooms.
- Daylight, white. This range is from 3500 to 5000 Kelvin. This glow is most similar to morning sunlight. 5000 K color temperature in the neutral range, recommended for use in bathrooms or hallways.
- Also, the 5000 K color range is used in educational institutions, offices, alleys, parks and production.
- Cold glow, white. The range of light is from 5000 Kelvin color temperature to 7000 K, temperature K 6500 is also included in this range.
Light temperature: definition, features and levels
The term “temperature of light” means, of course, not the actual temperature, but the color of light, or otherwise, the color gamut of light, the predominance of red or blue spectra in it.
Why know this?
It is important to know about color temperature for those who directly work with light, such as designers and photographers. Like no one else, they can confirm that the right color scheme of light can either completely transform everything (be it a person in the frame or the interior) or ruin it.
Pure black body
The temperature of the light source is measured in degrees Kelvin. It is calculated using Planck's formula: the temperature at which an absolutely black body will emit light of the same color tone, this will be the desired value.
Thus, color temperature is determined by comparing the desired light source with a completely black body. An interesting pattern: the higher the temperature of the latter, the more the blue spectrum predominates in the light.
The easiest way to observe in practice is: the color temperature of an incandescent lamp with warm white light is 2700 K, and a fluorescent fluorescent lamp is 6000 K. Why is this so? A completely black body can be compared to iron that is heated in a forge.
We all remember that a metal that is hot, but still at a fairly low temperature, has a red light, and we have often seen in the literature the expression “white-hot” - that is, to a much higher temperature.
Likewise, a black body emits light in this order of colors from red, orange and white, and ends with white and blue. That is, the lower the temperature of the light, the warmer it is.
Some meanings
The visible spectrum of a red-hot body, that same “red-hot” metal, starts at 800 degrees Kelvin. It's a dull, dark red glow. The yellow light of the flame has twice the temperature, from 1500 to 2000 K.
Lamps that are usually used for filming produce readings of about 3250 degrees. The sun, setting towards the horizon, shines with a temperature of 3400 K, and the temperature of daylight is almost 5000 K. The color temperature of the flash light is 5500-5600 degrees.
Lamps with multilayer phosphor, depending on the light bin, have indicators from 2700 to 7700 K.
Interesting paradoxes
Thus, the word “temperature” here acts as a determinant of color. At first it will be difficult to get used to the fact that the temperature of a clear blue sky (12,000 K) is ten times (!) higher than the temperature of the flames of a fire (1200 K). And in the region of the poles the sky is even “warmer” - about 20,000 K! The temperature of sunlight fluctuates during the day from 3000 to 7000 K.
It also attracts attention that different shades have different luminous intensities, that is, they spread differently. It would be incorrect to cite as an example a candle flame, which illuminates only a small fraction of the space around it, and a white LED, which is much brighter, but you can compare two identical LEDs of yellow and white colors. Despite the identical size and power, the yellow LED is dimmer, and the red LED illuminates even worse.
Gradations
We often encounter shades of the same color. In lighting technology, these are most often gradations of white: cold, neutral and warm. In fact, even such minor changes in the nature of the gamma affect such a delicate and precise instrument as the human eye. These shades of white not only convey the color of illuminated objects differently, but also behave differently in different weather conditions, and the range of their light beam also differs.
All of the above features are taken into account by modern manufacturers when creating certain lighting devices, but in order to understand the difference with colors, you need to introduce another important parameter.
Color rendition
The light temperature of the lamps is not the only thing you should know. Another fundamental term in lighting technology is color rendition. Surely everyone has seen more than once that, depending on the lighting, we can perceive the same color differently.
Yes, the names of colors are just an agreement between people to designate with a certain word one or another wavelength that we perceive. In fact, our eye distinguishes about ten million different shades, but we see most of them in daylight, sunlight.
It is accepted as the standard.
Thus, color rendering, or the degree of overall color rendering coefficient, is the correspondence of a light source to a standard or the ability to convey the color of an illuminated object in the same way as in sunlight. Measured in Ra, the term color rendering index is also used - CRI, color rendering index.
The standard has a value equal to 100 Ra (or CRI), and the lower this indicator is for a lamp or lantern, the worse this light conveys the natural shade of the object.
Best options
Temperature, light, humidity are the most important indicators of comfort in any room, so it is important to choose the right shade for lighting. The temperature of lamps and LED lights with cool white light ranges from 5000 to 7000 K.
Cool white, as it is called according to the manufacturers' markings, has a fairly low color rendering index, only about 60-65, that is, in this light the human eye perceives colors differently: perhaps everyone has noticed how much everything changes in a “lifeless” pale blue light.
However, it has the highest contrast among all shades, which means it is indispensable when illumination of dark-colored objects is required (for example, wet asphalt, earth). Another feature is its long-range efficiency, which is why the “cool white” shade is usually used in long-range flashlights (flow range is about 200 m).
A neutral white LED - neutral white - has a temperature ranging from 3700 to 5000 K. Its CRI is about 75, which means that color rendition is an order of magnitude higher than a cold bin. However, the range of the light beam is lower, so lanterns with neutral white light have a much shorter distance, but are more comfortable for the eyes.
The temperature of warm light (warm white) is from 2500 to 3700 K. The color perception index is even higher, about 80, but the range is even less than that of a neutral bin. However, warm and neutral shades have an advantage over cold white if lighting is needed in conditions of high smoke, humidity (rain, fog), as well as under water if it contains suspension (for example, in ponds). In such situations, cool white illuminates much more not the object itself, but the space before it, forming a pipe of light.
What is color temperature
Connecting LED lamps
When heated, all bodies emit light: first infrared, and then visible. The spectrum of this radiation can be used to determine body temperature. It is measured in Kelvin (K).
Conversely, each shade of radiation color corresponds to the temperature of the object. Therefore, shades of white are usually designated in Kelvin, so as not to come up with definitions like “light yellow” or “white with a blue tint”:
- 0°K – absolutely black body, absence of any radiation;
- 800°K (527°C) – dark red color;
- 1300°K (1027°C) – bright red. This is how heated metal glows;
- 2000°K (1727°C) – orange. This is the color of the coals (not the flames) in the fireplace;
- 2700°K – warm white color. This is how incandescent light bulbs glow;
- 4500°K – neutral white. The color of a cloudy day;
- 5000°K – white. This shade has the color of a sunny afternoon;
- 6800°K – cool white. Lighting at sunrise;
- 9000°K – blue. Thermonuclear reaction color.
Color temperature in Kelvin
At what temperature does iron turn red?
HEAT COLORS – colors of metal glow, depending on the heating temperature. Heat colors characteristic of steel, see table Temperature, ° C Heat color 550 Dark brown 630 Brown red 680 Dark red 740 Dark cherry 770 Cherry 800 Bright or light cherry 850 Bright or light red 900 Bright -red 950 Yellow-red TEN COLORS – iridescent coloring that appears on the clean surface of heated metal as a result of the appearance of a thin layer of oxides on it. colors of tarnish characteristic of carbon steel, see in the table Temperature, °C Color of tarnish 220 Straw 230 Golden 240 Brown 250 Red-brown 260 Purple 280 Violet 300 Blue (cornflower blue) 320 Light blue 330-350 Light gray On alloy steels these Tarnish colors appear at higher temperatures.
Well, judging by the heat in the stove, it’s 150 degrees
It is not clear why the answer is given about metal when the question is about iron. And there is a wonderful anecdote on this topic: - Comrade soldiers, let's unload the luminaries, let's go! - Sorry, comrade warrant officer, not lumine, but aluminum. - And they are extremely competent, for unloading cast iron, for leeeee! And someone else chose the best answer. And this, as they say, “I’m talking about Thomas to you, and you’re talking to me about Yerema.” So what is the temperature of red-hot iron?
Application[edit | edit code]
Tarnish colors most often occur during oxidation, as a result of heat treatment of metals. Usually, with rapid heating, they quickly replace each other, in a typical sequence: light straw, gold, purple, violet, blue, and then, as the thickness of the film increases, they reappear, but in a somewhat muted form: brownish yellow, red…
The color of tarnish, as well as the color of heat - the glow of metal heated to high temperatures (for example, for steel from dark brown at 550 ° C to white at 1300 ° C) in the past, before the advent of pyrometers, was widely used as an indicator of the heating temperature of iron and steel during heat treatment. The color of tarnish was also used to judge the heating temperature of steel chips, and, consequently, of the cutter during turning, drilling, and cutting operations.
Tarnish colors are not a very accurate indicator. They are significantly influenced by the composition of the alloy, the rate of temperature rise, the composition of the gaseous medium, the time the steel is held at a given temperature, as well as the nature of lighting and other factors.
On alloy steels, tarnish colors usually appear at higher temperatures, since alloying generally increases the steel's resistance to oxidation in air.
Tarnish colors are used for decorative finishing of steel products, as well as for their laser marking.
Approximate tarnish colors for steel edit | edit code
Carbon steel is characterized by the following color transitions: straw (220 °C), brown (240 °C), purple (260 °C), blue (300 °C), light gray (330-350 °C).
| Temperature, °C | Tarnish colors |
| 200 | Light straw |
| 220 | Straw |
| 225 | Light yellow |
| 230 | Golden |
| 240 | Brown-yellow |
| 255 | Brown |
| 260 | Red-brown |
| 270 | Purple red |
| 280-285 | Violet |
| 295-300 | Bright blue (cornflower blue) |
| 310 | Light blue |
| 320-325 | Light blue |
| 330-350 | Light gray |
On alloy steels, these tarnish colors appear at higher temperatures.